Design
Project
Contents
Introduction
Sense of hearing
As found in human ears, mechanosensitive (MS) channels take responsibility for the sense of hearing. [1]
In plants, some cell membrane-associated proteins (also belonging to the MS channels) are proposed to be associated with sound sensing. They are proved to be sensitive towards sounds with specific frequency and intensity. [2]
Some research have been made in discovering the relationship between MS channels and sound. Most of researchers employed ultrasound, which might cause cell damage during experiments. [3][4]
It is hard to explain how the ears can hear sound by sensing weak and low-frequency vibration. In our project, we chose two kinds of MS channels, transient receptor potential channel 5 (TRPC5) and Piezo1 as research objects. Ultrasound as well as audible sound were used to test the response of both MS channels. We hoped we could make some contribution to exploring the secret of hearing and help hearing-impaired person. Additionally, we expected to select some mutated MS channels that are sensitive to distinctive sound frequency.
Gene regulation
Gene expression is a complex and stochastic process involving numerous processes and various reaction steps. [5]
With continuing development in synthetic biology, a large amount of methods are developed to regulate gene expression artificially, including the most common two regulation systems, chemical genetics [1] and optogenetics[6][7].
In chemical genetics, small molecular drugs such as aTc are used to induce gene expression, while it lacks spatial specificity and the concentration of small chemicals in the cell is not stable. Optogenetics employs light as input signal to induce gene expression. However, there are side effects caused by laser-induced heating and abnormal ion distribution caused by overexpressed pumps or channels. In addition, undesired disturbance of homeostasis can make it very difficult for experimental interpretation [8].
Based on the previous study, we hoped to find out a mechanical channel which can respond to specific sound wave and to realize audiogenetics. Compared to the methods above, sound signal is easier to generate and less harmful to research objects.
Study overview
In our study, audiogenetics is defined as a method of gene regulation by applying sound precisely and safely. The external sound signal is transduced through two mechanosensitive channels, TRPC5 [9] [10] [11] and Piezo1[12][13].
Different from other MS channels, these two channels open the gate to allow calcium ions selectively permeate through cell membrane when mechanical force is applied.[14] The influx of calcium promotes the activation of TRPC5 channel and more calcium will influx into cytoplasm [15]. Since Piezo1 channel has been proved more sensitive to mechanical force, it was involved in our study as a test component for audiogenetics study. Meanwhile, the structure of TRPC5 is better studied than Piezo1, therefore we applied directed evolution within its ankyrin repeats region to enhance channel sensitivity.
To quantitatively measure intracellular calcium level in an instant manner, we employed a fluorescent indicator, R-GECO. It is an artificial calcium indicator derived from GCaMP. After binding with calcium, it emits red fluorescence at around 600nm [16].
As to long term calcium indicator, since cytoplasmic calcium regulates a series of phosphorylation, we chose a specific promoter (PNFAT) as an inductive element to regulate the downstream transgene expression. Green fluorescent protein (GFP) was employed as the output signal to help us quantitatively analyze the regulatory ability of our audiogenetic system [17].
We tested the system above in CHO-K1 cell line, because it doesn’t normally express mechanosensitive channel activity. [18] [19] Also it is convenient for us to test the channel’s response to sound wave in different cases. Moreover, to quantify the sensitivity of Piezo1 and TRPC5 to mechanical stress, we firstly active MS channels by microfluidics and hypoosmolarity [20]to observe the calcium influx level. Then, we applied sounds with various frequencies and intensities by using self-designed sound generators such as buzzer, balanced armature, horn, ultrasound and atomizer.
The successful audiogenetics will reshape the landscape of current methods for gene regulation. This novel technology will open a new door to audio control of gene expression, neuronal activities and other biological process in cells or organisms.
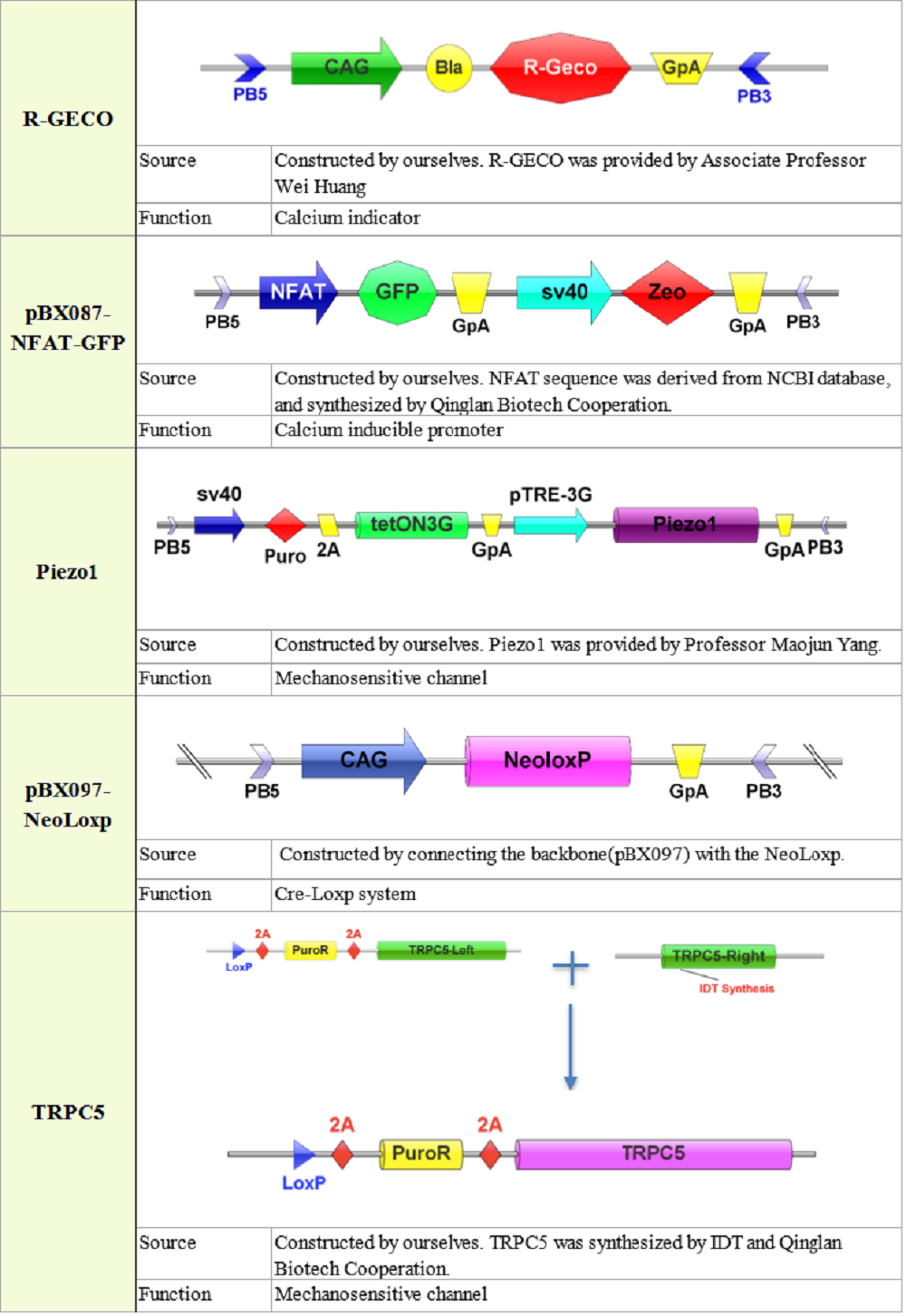
Plasmid construction
Measurement
Quantitatively examine the function of R-GECO
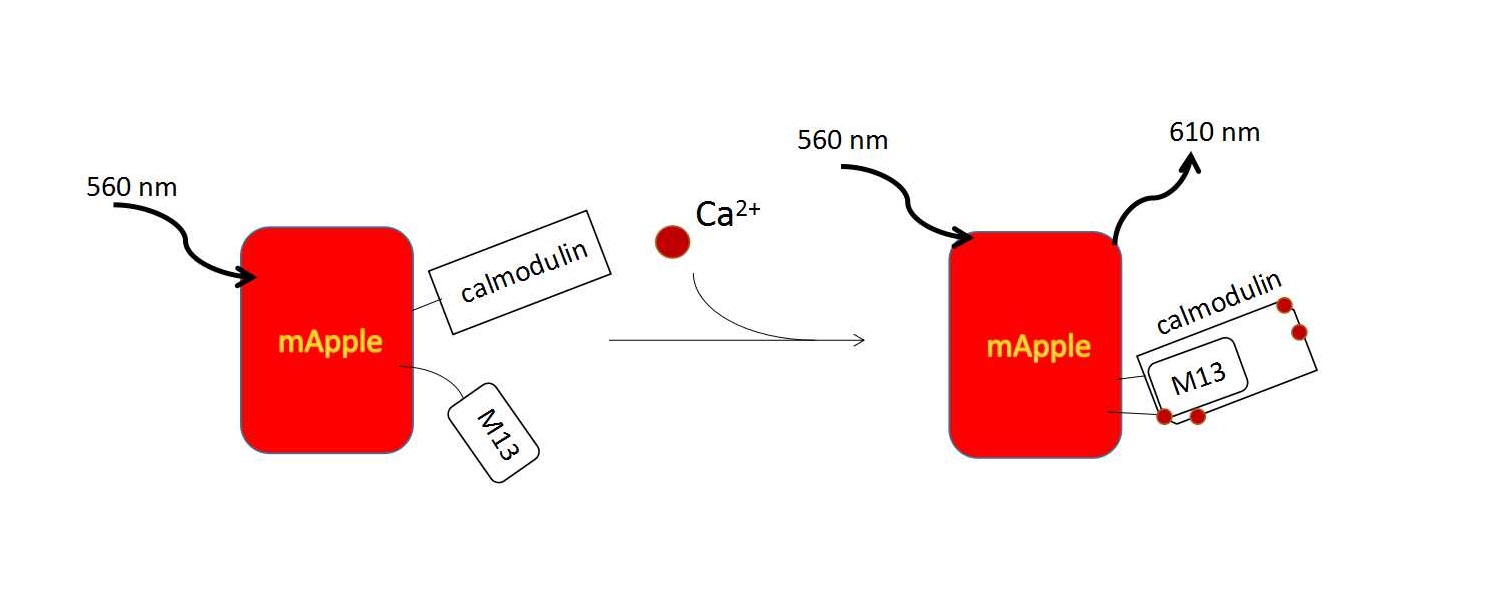
GCaMPs consists of a circularly permuted enhanced green fluorescent protein (such as EGFP), which is flanked on one side by the calcium-binding protein calmodulin and on the other side by the calmodulin-binding peptide M13. In the presence of calcium, calmodulin-M13 interactions elicit conformational changes in the fluorophore environment that leads to an increase in the emitted fluorescence. (Fig. 2). [21] With a series of artificial modification, a group of calcium indicators, GECO family was created. They are useful in neuronal activity study and cell imaging. We could use following methods to induce calcium influx to quantitatively examine the function of R-GECO.
Mechanism of R-GECO
Depolarization
Potassium ion is involved to make a depolarization and lead the increases in cytosolic free calcium.[22]
Ionomycin
Ionomycin is an ionophore produced by the bacterium Streptomyces conglobatus. It is used in research to raise the intracellular level of calcium (Ca2+) by stimulating store-regulated cation entry.[23]
Quantitatively examine the sensibility of Piezo1 and TRPC5
Transient receptor potential (TRP) channels belong to a diverse family of cation channels that respond to a variety of signals.[24][25] For example, some are involved in sensory perception, directly activated by chemical ligands and physical stimuli such as temperature, mechanical and osmotic stresses. Others are activated by downstream receptor stimulation through phospholipase C (PLC)-dependent pathway.
TRPC5 is a calcium permeable cation channel predominantly expressed in the central nervous system (CNS). There is an impressive array of other activators of TRPC5 channels, such as nitric oxide, lysophospholipids, sphingosine-1-phosphate, reduced thioredoxin, protons, lanthanides, and calcium, and many can cause the change of its configuration. Moreover, TRPC5 shows constitutive activity, and it is shown to be associated with membrane stretch and cold feeling. Thus, TRPC5 channels have significant potential for synergistic activation and may serve as an important focal point in calcium signaling and electrogenesis. The biological functions of TRPC5 channel are also significant, ranging from neurotransmission to control of axon guidance and vascular smooth muscle cell migration and contractility.[26]
Piezo1 protein is a functionally diverse mechanosensitive cation channel. It is expressed in lungs, bladder and skin, where mechanosensation plays an important biological role. It also plays significant role in multiple physiological processes, including sensing shear stress of blood flow for proper blood vessel development, regulating red blood cell function and controlling cell migration as well as differentiation.[27]
Microfluidics
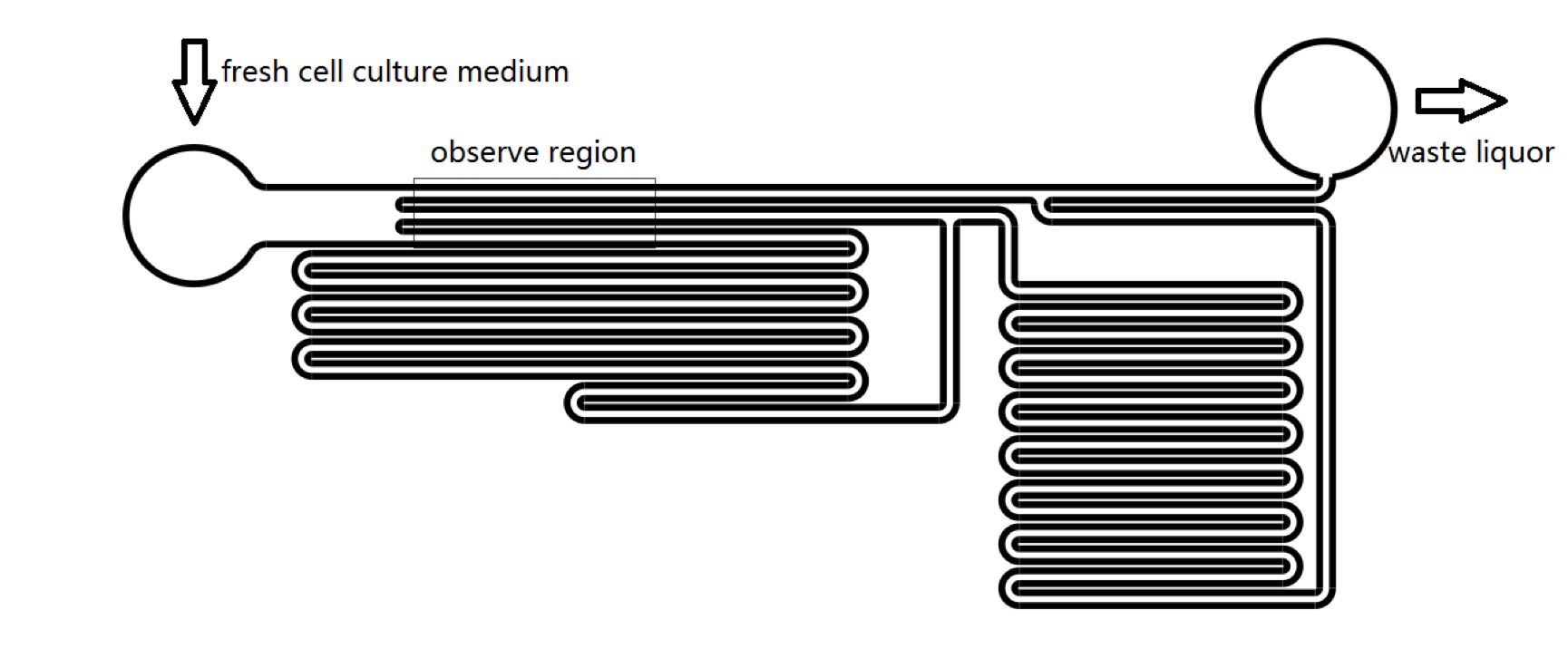
We used microfluidic devices to exert shear stress to stimulate Piezo1 and TRPC5 channels. We could control the pressure on cell membrane by changing the velocity of cell culture medium flowing in microfluidic chips. It takes only 1 second for R-GECO to reach maximum fluorescence intensity. Thus we could see whether Piezo1 and TRPC5 can be activated by mechanical stress instantaneously.We could also examine in sensitivity of MS channels in terms of mechanical stress (Fig. 3). In addition, we could calculate how much pressure should be added on cell’s membrane to activate the MS channels, which was quite important for our sound generator experimental design (See Model).
Hypoosmolarity
In addition, we used osmotic pressure to exert mechanical stress on cell plasma membrane to activate Piezo1 or TRPC5. The result turned out that the cells could sense this stress, which means that hypoosmolarity can trigger calcium influx through Piezo1 and TRPC5.
Quantitatively analyzes the regulatory ability of audiogenetics
Firstly, we used some self-made sound generators such as buzzer, balanced armature, horn, ultrasound and atomizer to activate Piezo1 and TRPC5 channel and observe the calcium influx. Since downstream GFP expression induced by calcium signal needs about 24 hours to reach maximum level, we could use long term pulse sound to stimulate the MS channels. Then we could quantitatively analyze the regulatory ability of audiogenetics. The designed audio generators need to be convenient, controllable and suitable for cell culturing. Also we need to calculate how much energy we should apply to exert pressure on cell’s membrane. The formula of sound intensity and its corresponding pressure on cell is below. P is pressure (N/m2), I is sound intensity (W/m2), ρ is medium density and c is sound velocity.
I = \frac{P^{2}}{2c\rho}
Directed evolution of TRPC5
TRPC5 structure
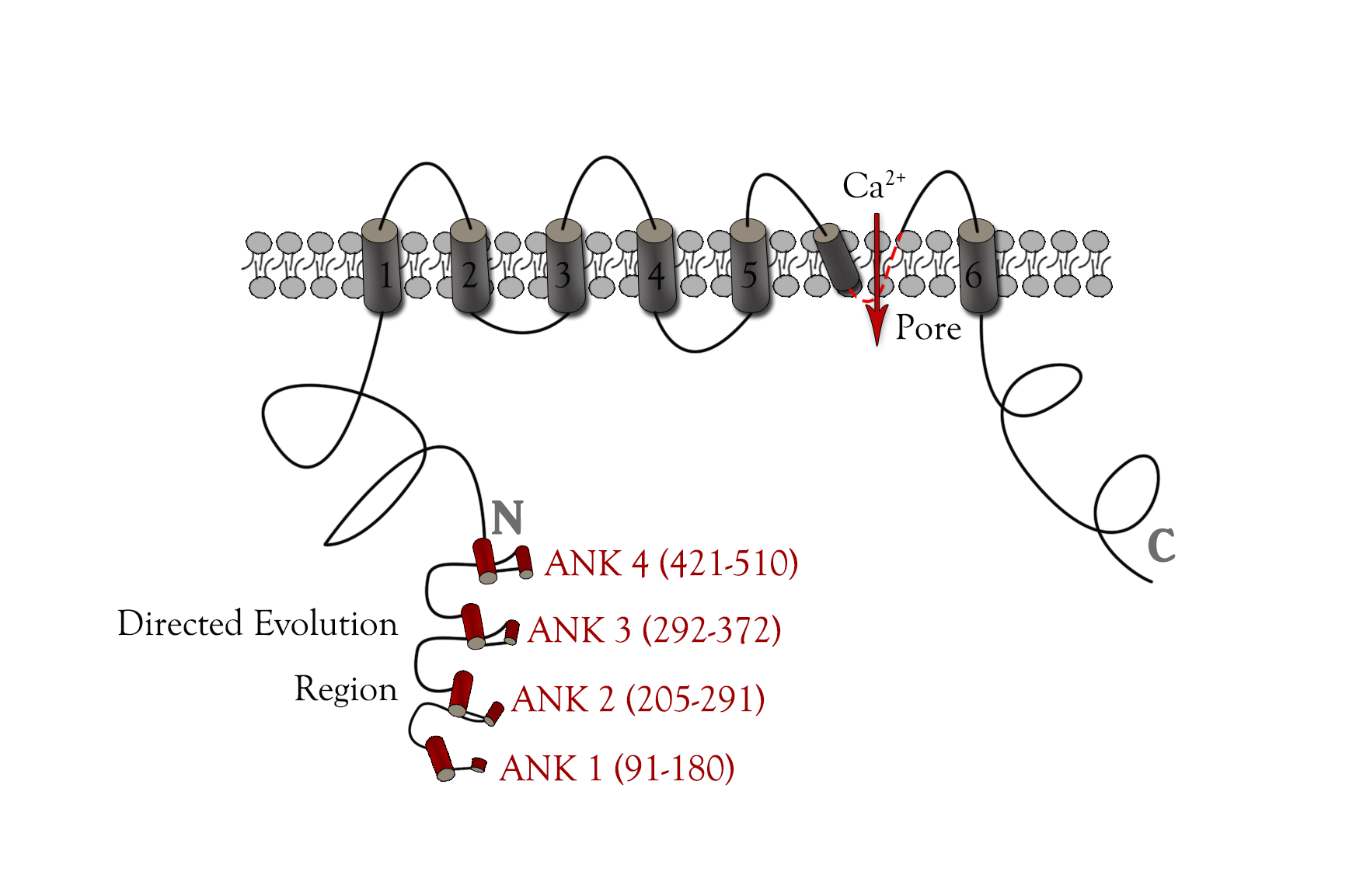
In order to get mutated TRPC5 with high sensitivity to mechanical stress, we need to find out the specific fragment of TRPC5 which is related to stress sensing. Many researches have revealed the structure of TRPC5.[28][29][30] (Fig. 4) Based on their study, we knew the ankyrin repeats[31], S4-S5 linker and pore region[32] were crucial positions for TRPC5 directed evolution.
However, mutation at S4-S5 linker or pore helix would cause continuously Ca2+ influx and cell death, so it might not be appropriate to mutate at these transmembrane protein areas. Crystallographic studies have shown that multiple ankyrin repeats could form a helical structure, which might act as a gating spring connected to cytoskeletal elements,[33] [34]so we finally chose the ankyrin repeats region as the mutated region.
Library construction
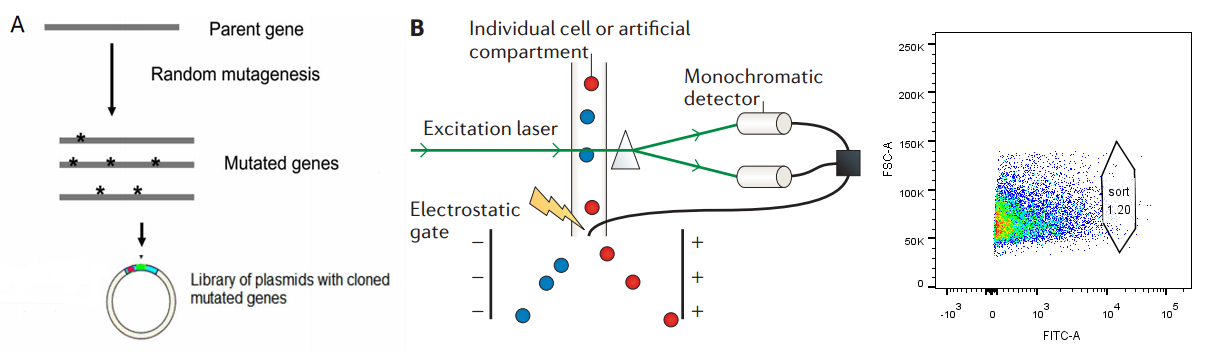
We used GeneMorph II Random Mutagenesis Kit (Provided by Agilent Technologies Company) to make a library of mutated ankyrin repeats by error-prone PCR. (Fig. 5a) The GeneMorph II kit has been used to mutagenize plasmid DNA targets up to 6 kb in length. Its mutation rate can be controlled simply by varying the initial amount of target DNA in the reaction or the number of amplification cycles performed. This kit also allows researchers to choose the mutation frequency that is most appropriate for a particular application.
In directed evolution studies, mutation frequencies of 1~4 amino acid changes (2~7 nucleotide changes) per gene are commonly employed. Proteins with improved activities have also been isolated from highly mutagenized libraries exhibiting 20 mutations per gene.
Using the mutated (Ankyrin repeats) fragments, we compared the efficiency of three different ways (whole plasmid mutagenesis, Gibson assembly, and ligation) to construct the mutated Ankyrin repeats fragments into intact plasmids.
For whole plasmid mutagenesis, it uses a pair of complementary mutagenic primers (the mutated Ankyrin repeats fragments) to amplify the entire plasmid in a thermocycling reaction using high-fidelity non-strand-displacing DNA polymerase such as Q5 polymerase, and the reaction generates a nicked, circular DNA. Gibson assembly is a method to combine more than ten DNA fragments based on sequence identity. DNA fragments are required to contain around 20 to 40 base pairs overlapping with adjacent DNA fragments. Ligation is a method using T4 ligase to join double strand DNA fragments with the same sticky end.
Through experiments in different groups, we found out the ligation method is the most efficient way to construct mutation library, with ligation efficiency of 0.8%.
Screening
To screen out the desired TRPC5 mutant with high sensitivity to mechanical stress, we first transfected the CHO-K1 cells with a large library of TRPC5 mutant mixture cloned in the same plasmid backbone. Then we used fluorescence activated cell sorting (FACS) to select the cells with proper intensity of GFP (Fig. 5b), since TRPC5 channel with high sensitivity would induce more calcium influx into cytosolic under the same sound wave signal, and the high concentration of free calcium in cytosolic would induce more GFP expression through downstream promoter (pNFAT). We need to ensure each transfected cell using PiggyBac system contains only one mutated TRPC5. Otherwise, cells contain two or more copy number of TRPC5 would be more sensitive to mechanical stress due to superimposed effect.
First, we use Cre-Loxp system to select the single cell colony only with one copy number of Loxp after transfected by PiggyBac mediated multiplex gene transfer method,[35] which can be done through qPCR. Then we transfect mutated TRPC5 with Loxp sequence ahead into cells. Therefore, all cells will contain one copy of mutated TRPC5, besides, it is in the same position of CHO-K1’s genome. In the end, we use vibration to stimulate transfected CHO-K1 cell that cultured in flask.
References
- ↑ 1.0 1.1 Nevozhay, D., et al., Negative autoregulation linearizes the dose-response and suppresses the heterogeneity of gene expression. Proc Natl Acad Sci U S A, 2009. 106(13): p. 5123-8.
- ↑ Qin, Y.C., et al., Biochemical and physiological changes in plants as a result of different sonic exposures. Ultrasonics, 2003. 41(5): p. 407-11.
- ↑ Haswell, E.S., R. Phillips, and D.C. Rees, Mechanosensitive channels: what can they do and how do they do it? Structure, 2011. 19(10): p. 1356-69.
- ↑ Mishra, R.C., R. Ghosh, and H. Bae, Plant acoustics: in the search of a sound mechanism for sound signaling in plants. J Exp Bot, 2016. 67(15): p. 4483-94.
- ↑ Ozbudak, E.M., et al., Regulation of noise in the expression of a single gene. Nat Genet, 2002. 31(1): p. 69-73.
- ↑ Leifer, A.M., et al., Optogenetic manipulation of neural activity in freely moving Caenorhabditis elegans. Nat Methods, 2011. 8(2): p. 147-52.
- ↑ Ye, H., et al., A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science, 2011. 332(6037): p. 1565-8.
- ↑ Hausser, M., Optogenetics: the age of light. Nat Methods, 2014. 11(10): p. 1012-4.
- ↑ Zholos, A.V., Trpc5. Handb Exp Pharmacol, 2014. 222: p. 129-56.
- ↑ Vazquez, G., et al., The mammalian TRPC cation channels. Biochim Biophys Acta, 2004. 1742(1-3): p. 21-36.
- ↑ Song, M.Y. and J.X. Yuan, Introduction to TRP channels: structure, function, and regulation. Adv Exp Med Biol, 2010. 661: p. 99-108.
- ↑ Ge, J., et al., Architecture of the mammalian mechanosensitive Piezo1 channel. Nature, 2015. 527(7576): p. 64-9.
- ↑ Pathak, M.M., et al., Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci U S A, 2014. 111(45): p. 16148-53.
- ↑ Inoue, R., Z. Jian, and Y. Kawarabayashi, Mechanosensitive TRP channels in cardiovascular pathophysiology. Pharmacol Ther, 2009. 123(3): p. 371-85.
- ↑ Ye, H., et al., A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science, 2011. 332(6037): p. 1565-8.
- ↑ Zhao, Y., et al., An expanded palette of genetically encoded Ca2+ indicators. Science, 2011. 333(6051): p. 1888-91.
- ↑ Ye, H., et al., A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science, 2011. 332(6037): p. 1565-8.
- ↑ Shen B, Wong C-O, Lau O-C, Woo T, Bai S, Huang Y, et al. (2015) Plasma Membrane Mechanical Stress Activates TRPC5 Channels. PLoS ONE 10(4):e0122227.
- ↑ Flockerzi, V., Nilius, B. (2007). TRPC Channel Subfamily. Transient Receptor Potential (TRP) Channels (pp. 42). America: Springer Science & Business Media.
- ↑ Shen, B., et al., Plasma membrane mechanical stress activates TRPC5 channels. PLoS One, 2015. 10(4): p. e0122227.
- ↑ Zhao, Y., et al., An expanded palette of genetically encoded Ca(2)(+) indicators. Science, 2011. 333(6051): p. 1888-91.
- ↑ Ranade, S.S., et al., Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci U S A, 2014. 111(28): p. 10347-52.
- ↑ Morgan, A.J. and R. Jacob, Ionomycin enhances Ca2+ influx by stimulating store-regulated cation entry and not by a direct action at the plasma membrane. Biochem J, 1994. 300 ( Pt 3): p. 665-72.
- ↑ Flockerzi, V., An introduction on TRP channels. Handb Exp Pharmacol, 2007(179): p. 1-19.
- ↑ Ramsey, I.S., M. Delling, and D.E. Clapham, An introduction to TRP channels. Annu Rev Physiol, 2006. 68: p. 619-47.
- ↑ Zholos, A.V., Trpc5. Handb Exp Pharmacol, 2014. 222: p. 129-56.
- ↑ Ge, J., et al., Architecture of the mammalian mechanosensitive Piezo1 channel. Nature, 2015. 527(7576): p. 64-9.
- ↑ Zholos, A.V., Trpc5. Handb Exp Pharmacol, 2014. 222: p. 129-56.
- ↑ Beck, A., et al., Conserved gating elements in TRPC4 and TRPC5 channels. J Biol Chem, 2013. 288(27): p. 19471-83.
- ↑ Gaudet, R., TRP channels entering the structural era. J Physiol, 2008. 586(15): p. 3565-75.
- ↑ Owsianik, G., et al., Structure-function relationship of the TRP channel superfamily. Rev Physiol Biochem Pharmacol, 2006. 156: p. 61-90.
- ↑ Beck, A., et al., Conserved gating elements in TRPC4 and TRPC5 channels. J Biol Chem, 2013. 288(27): p. 19471-83.
- ↑ Shen, B., et al., Plasma membrane mechanical stress activates TRPC5 channels. PLoS One, 2015. 10(4): p. e0122227.
- ↑ Ramsey, I.S., M. Delling, and D.E. Clapham, An introduction to TRP channels. Annu Rev Physiol, 2006. 68: p. 619-47.
- ↑ Lu, X. and W. Huang, PiggyBac mediated multiplex gene transfer in mouse embryonic stem cell. PLoS One, 2014. 9(12): p. e115072.