Cockroach627 (Talk | contribs) |
Cockroach627 (Talk | contribs) |
||
| Line 54: | Line 54: | ||
|- | |- | ||
| PstⅠ-1 | | PstⅠ-1 | ||
| − | |{{SUSTech_Image | filename=T--SUSTech_Shenzhen--qwe.png| caption=}} | + | |{{SUSTech_Image | filename=T--SUSTech_Shenzhen--qwe.png| caption= | width=700px}} |
|- | |- | ||
| PstⅠ-2 | | PstⅠ-2 | ||
| − | |{{SUSTech_Image | filename=T--SUSTech_Shenzhen--234444.png| caption=}} | + | |{{SUSTech_Image | filename=T--SUSTech_Shenzhen--234444.png| caption= | width=700px}} |
|- | |- | ||
| EcoRⅠ | | EcoRⅠ | ||
| − | |{{SUSTech_Image | filename=T--SUSTech_Shenzhen--444365.png| caption=}} | + | |{{SUSTech_Image | filename=T--SUSTech_Shenzhen--444365.png| caption= | width=700px}} |
|} | |} | ||
Revision as of 09:31, 19 October 2016

Part Collection
Contribution
Contents
Our Part Collection includes:
- Original TRPC5 plasmid (<partinfo>BBa_K1943021</partinfo>);
- Five mutated TRPC5 plasmids(<partinfo>BBa_K1943022</partinfo>, <partinfo>BBa_K1943023</partinfo>, <partinfo>BBa_K1943024</partinfo>, <partinfo>BBa_K1943025</partinfo>, <partinfo>BBa_K1943026</partinfo>).
Transient receptor potential channel 5 (TRPC5) is a mechanosensitive (MS) channel [1] which takes charge of hearing in our project. Future teams could use original TRPC5 plasmid (<partinfo>BBa_K1943021</partinfo>) to sense sound or other kinds of mechanical forces. Furthermore, we randomly mutated the ankyrin region of TRPC5 channel by PCR. We expected the ankyrin region to be a crucial part for sense mechanical forces based on the structure of TRPC5.[2](Detailed methods are shown in our notebook.)
These mutated TRPC5 plasmids(<partinfo>BBa_K1943022</partinfo>, <partinfo>BBa_K1943023</partinfo>, <partinfo>BBa_K1943024</partinfo>, <partinfo>BBa_K1943025</partinfo>, <partinfo>BBa_K1943026</partinfo>) have all been sequenced. Because of limited time, we have not yet transfected them into cells to observe whether the channels are more sensitive to sound and mechanical force or not. Future teams are welcomed to use and finish testing them.
The mutated amino acid sites are as listed:
| Part | Original amino acids | Amino acid after mutation |
|---|---|---|
| <partinfo>BBa_K1943022</partinfo> | Trs(240)+Ala(27) | Glu(240)+Thr(27) |
| <partinfo>BBa_K1943023</partinfo> | Ala(27) | Thr(27) |
| <partinfo>BBa_K1943024</partinfo> | Met(134)+Ala(27) | Ile(134)+Thr(27) |
| <partinfo>BBa_K1943025</partinfo> | Glu(236)+Ala(27) | Lys(236)+Thr(27) |
| <partinfo>BBa_K1943026</partinfo> | Gln(128)+Ala(27) | Arg(128)+Thr(27) |
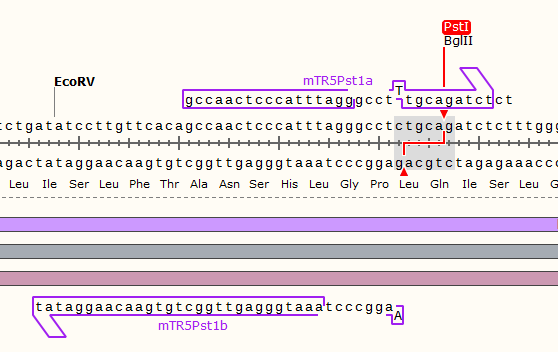
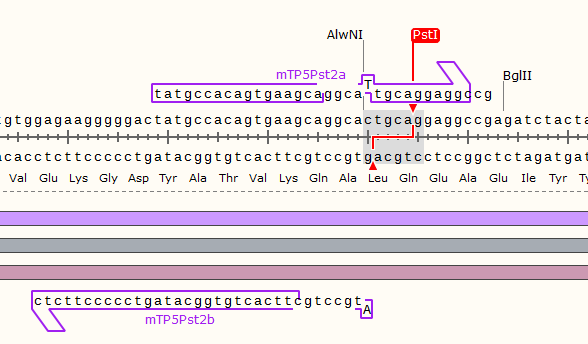
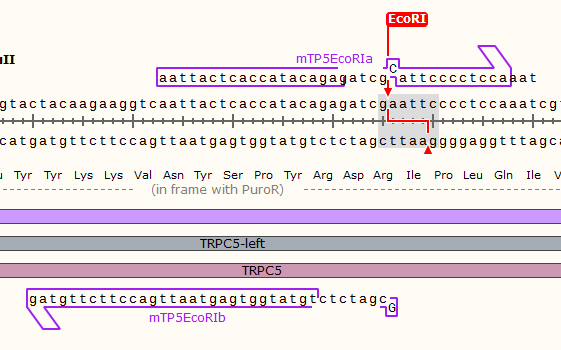
TRPC5 sequence contains 1 EcoRⅠ RE site and 2 PstⅠ RE sites, which are unacceptable for BioBricks. Therefore, we did site-mutation by PCR for three times. Detailed information are shown in our notebook.
Site-directed mutation experiments
The primers we used are as listed:
| Mutated RE sites | Primer design |
|---|---|
| PstⅠ-1 | |
| PstⅠ-2 | |
| EcoRⅠ |
References:
- ↑ Sossey-Alaoui K., et al, Molecular cloning and characterization of TRPC5 (HTRP5), the human homologue of a mouse brain receptor-activated capacitative Ca2+ entry channel., Genomics, Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10493832.
- ↑ Vazquez, G., et al., The mammalian TRPC cation channels., Biochim Biophys Acta, Retrieved from http://www.sciencedirect.com/science/article/pii/S0167488904002101.