| September |
|
|
1 |
2 |
3 |
4 |
5 |
| 6 |
7 |
8 |
9 |
10 |
11 |
12 |
| 13 |
14 |
15 |
16 |
17 |
18 |
19 |
| 20 |
21 |
22 |
23 |
24 |
25 |
26 |
| 27 |
28 |
29 |
30 |
|
|
|
|
|
|
|
8/14
Transformation
Li, Uchino, Kaneko
| Sample Name | Sample Volume (µl) | Competent Cells/(µl) | Medium |
|---|
| RFP 0.5ng/µl | 2 | DH5α /20 | LB(CP) |
| RFP 5ng/µl | 2 | DH5α /20 | LB(CP) |
| RFP 10ng/µl | 2 | DH5α /20 | LB(CP) |
| RFP 20ng/µl | 2 | DH5α /20 | LB(CP) |
| RFP 50ng/µl | 2 | DH5α /20 | LB(CP) |
8/14
Gel Extraction
Li, Uchino, Kaneko
| Lane | Restriction Enzyme Digestion Product | Volume/(µl) |
|---|
| 1 | 10/1 PQ(3) | about 23.5 |
| 2 | 1kb ladder | 6 |
| 3 | 10/1 PQ(3) | about 23.5 |
| 4 | 10/1 PQ(3) | about 24 |

Total volume of samples of lane 1~3 was 71.5µl and it was divided into three.
8/14
Transformation
Li, Uchino, Kaneko
| Sample Name | Sample Volume | Competent Cells/(µl) | Medium |
|---|
| 3C5 | 10µl | DH5α 20µl | LB(CP) |
| P+ | 10µl | DH5α 20µl | LB(CP) |
| P- | 10µl | DH5α 20µl | LB(CP) |
| RBS | 10µl | DH5α 20µl | LB(CP) |
8/14
Liquid Culture
Li, Uchino, Iguchi, Kaneko
| Name | medium |
|---|
| 8/13 transformation RFP 20ng/µl colony1 | LB(CP) |
| 8/13 transformation part RFP colony1 | LB(CP) |
| 8/13 transformation part RFP colony2 | LB(CP) |
| 8/13 transformation part RFP colony3 | LB(CP) |
| 8/13 transformation part RFP colony4 | LB(CP) |
| 8/13 transformation part RFP colony5 | LB(CP) |
| 8/13 transformation part RFP colony6 | LB(CP) |
| 8/13 transformation part RFP colony7 | LB(CP) |
| 8/13 transformation part RFP colony8 | LB(CP) |
| 8/13 transformation part RFP colony9 | LB(CP) |
| 8/13 transformation part RFP colony10 | LB(CP) |
| 8/13 transformation part RFP colony11 | LB(CP) |
8/15
Miniprep
Li, Uchino, Notsu, Kaneko, Watanabe
| DNA | Concentration/(µg/ml) | 260/280 | 260/230 |
|---|
| RFP1 /2µl | 82.5 | 1.40 | 1.22 |
| RFP2 /4µl | 142.5 | 1.69 | 2.05 |
| RFP3 /4µl | 50 | 1.55 | 1.71 |
| RFP4 /4µl | 57.5 | 1.64 | 1.88 |
| RFP6 /4µl | 98.75 | 1.71 | 1.40 |
| RFP(20) /4µl | 76.25 | 1.67 | 1.32 |
8/15
Electrophoresis
Notsu, Kaneko
| Lane | Restriction Enzyme Digestion Products |
|---|
| 1 | 1kb ladder 3µl |
| 2 | RFP1 10µl |
| 3 | RFP2 10µl |
| 4 | RFP3 10µl |
| 5 | RFP4 10µl |
| 6 | RFP6 10µl |
| 7 | RFP(20) 10µl |
| 8 | PCR1 5µl |
| 9 | PCR2 5µl |
| 10 | PCR3 5µl |
| 11 | PCR4 5µl |
| 12 | PCR5 5 µl |
| 13 | 1kb ladder 3µl |

8/15
Restriction Enzyme Digestion
Uchino, Notsu, Kaneko
| Sample | DNA/(µl) | EcoR1/(µl) | Xba1/(µl) | Spe1/(µl) | Pst1/(µl) | Buffer/(µl) | BSA/(µl) | MilliQ/(µl) | Total/(µl) |
|---|
| 8/15Miniprep PSB1C3-RFP1 | 24 | 1 | 0 | 0 | 1 | 5(H) | 0 | 19 | 50 |
| 8/15Miniprep PSB1C3-RFP2 | 14 | 1 | 0 | 0 | 1 | 5(H) | 0 | 29 | 50 |
| 8/15Miniprep PSB1C3-RFP3 | 40 | 1 | 0 | 0 | 1 | 5(H) | 0 | 3 | 50 |
| 8/15Miniprep PSB1C3-RFP4 | 35 | 1 | 0 | 0 | 1 | 5(H) | 0 | 8 | 50 |
| 8/15Miniprep PSB1C3-RFP6 | 20 | 1 | 0 | 0 | 1 | 5(H) | 0 | 23 | 50 |
8/15
colony PCR
Uchino, Notsu, Kaneko, Watanabe
| colony Primers |
|---|
| 8/14 transformation pSB1C3-RFP1 VF+VR |
| 8/14 transformation pSB1C3-RFP2 VF+VR |
| 8/14 transformation pSB1C3-RFP3 VF+VR |
| 8/14 transformation pSB1C3-RFP4 VF+VR |
| 8/14 transformation pSB1C3-RFP1 VF+VR |
8/15
PCR (Steps)
Uchino, Notsu, Kaneko, Watanabe
| Steps | Temparature | Time | Cycle |
|---|
| Pre Denature | 94℃ | 2min | 1 |
| Denature | 94℃ | 30sec | 30 |
| Annealing | 55℃ | 30sec | 30 |
| Extension | 68℃ | 1min | 30 |
| Final elongation | 4℃ | 7min | 1 |
| Final Holding | 4℃ | - | - |
8/16
Gel Extraction
Li, Iguchi, Notsu, Kaneko, Watanabe
| Lane | Restriction Enzyme Digestion Product | Volume/(µl) |
|---|
| 1 | 1kb ladder | 10 |
| 2 | blank | |
| 3 | 8/15 digestion pSB1C3-RFP 1 | 55 |
| 4 | 8/15 digestion pSB1C3-RFP 2 | 55 |
| 5 | 8/15 digestion pSB1C3-RFP 3 | 36 |
| 6 | 8/15 digestion pSB1C3-RFP 4 | 36 |
| 7 | 8/15 digestion pSB1C3-RFP 6 | 36 |

8/16
Electrophoresis
Li, Iguchi, Notsu, Kaneko, Watanabe
| Lane | sample/(µl) | Length(bp) |
|---|
| 1 | 8/16 Gel Extraction RFP1 /1 | |
| 2 | 8/16 Gel Extraction RFP2 /1 | |
| 3 | 8/16 Gel Extraction RFP3 /1 | |
| 4 | 8/16 Gel Extraction RFP4 /1 | |
| 5 | blank | |
| 6 | 8/16 pSB1C3-vector /1 | |
| 7 | 8/16 pSB1C3-vector /1 | |
| 8 | 1kladder | |

8/16
Ligation
Li, Iguchi, Notsu, Kaneko, Watanabe
| Vector DNA /(µl) | insert DNA /(µl) | ligase /(µl) | 10×buffer /(µl) | MilliQ /(µl) | Total /(µl) |
|---|
| pSB1C3 EPcut /1 | RFP /4 | 0.5 | 1 | 3 | 9.5 |
| pSB1C3 EPcut /1 | RFP /2 | 0.5 | 1 | 5 | 9.5 |
| pSB1C3 EPcut /1 | RFP /4 | 0.5 | 1 | 3 | 9.5 |
| pSB1C3 EPcut /1 | RFP /4 | 0.5 | 1 | 3 | 9.5 |
| pSB1C3 EPcut /1 | - | 0.5 | 1 | 7 | 9.5 |
8/17
Liquid Culture
Li
| Name | medium |
|---|
| 8/16 transformation pSB3C5 RFP1 | LB(CP) /6 |
| 8/16 transformation pSB3C5 RFP2 | LB(CP) /6 |
| 8/16 transformation pSB3C5 RFP1 | LB(CP) /6 |
8/17
Transformation
Li
| Sample Name | Sample Volume (µl) | Competent Cells/(µl) | Medium |
|---|
| pSB1C3 P+ | 2 | DH5α /20 | LB |
| pSB1C3 P- | 2 | DH5α /20 | LB |
| pSB1C3 RBS | 2 | DH5α /20 | LB |
| pSB3C5 RFP | 2 | DH5α /20 | LB |
| 8/16 ligation pSB1C3 RFP1 | 2 | DH5α | LB |
8/17
Electrophoresis
Iguchi
| Lane | sample/(µl) | Length(bp) |
|---|
| 1 | 1kbp ladder /3 | |
| 2 | blank | |
| 3 | pSB1C3-RFP(ligate)1 /2 | |
| 4 | pSB1C3-RFP(ligate)2 /2 | |
| 5 | pSB1C3-RFP(ligate)3 /2 | |
| 6 | pSB1C3-RFP(ligate)4 /3 | |
| 7 | Vector (Ctrl) /5 | |

8/18
Liquid Culture
Li, Iguchi, Notsu
| Name | medium |
|---|
| INP [1] BBa_K811005 | LB(CP) / 6ml |
| INP [2] BBa_K811005 | LB(CP) / 6ml |
| INP [3] BBa_K811005 | LB(CP) / 6ml |
| scfv04 [1] BBa_K875004 | LB(CP) / 6ml |
| scfv04 [2] BBa_K875004 | LB(CP) / 6ml |
| scfv04 [3] BBa_K875004 | LB(CP) / 6ml |
| scfv06 [1] BBa__K875006 | LB(CP) / 6ml |
| scfv06 [2] BBa_K875006 | LB(CP) / 6ml |
| scfv06 [3] BBa_K875006 | LB(CP) / 6ml |
8/18
Miniprep
Li, Iguchi, Notsu
| DNA | Concentration/(µg/ml) | 260/280 | 260/230 |
|---|
| 8/17 Liquid Culture 3C5 [1] / 5µl | 77 | 1.69 | 1.45 |
| 8/17 Liquid Culture 3C5 [2] / 2µl | 40 | 1.67 | 1.17 |
| 8/17 Liquid Culture 3C5 [3] / 2µl | 15 | 3.75 | 3.70 |
8/18
Electrophoresis
Li
| Lane | sample/(µl) | Length(bp) |
|---|
| 1 | 1kb ladder /1 | - |
| 2 | [1] psb3c5(8/18 miniprep) /4.2 | 3807 |
| 3 | [2] psb3c5(8/18 miniprep) /4.2 | 3807 |
| 4 | [1'] psb3c5(8/18 miniprep) /4.2 | 3807 |
| 5 | [2'] psb3c5(8/18 miniprep) /4.2 | 3807 |
| 6 | [3'] psb3c5(8/18 miniprep) /4.2 | 3807 |

[img18]
8/18
Restriction Enzyme Digestion
Li, Iguchi, Notsu
| Sample | DNA/(µl) | EcoR1/(µl) | Xba1/(µl) | Spe1/(µl) | Pst1/(µl) | Buffer/(µl) | BSA/(µl) | MilliQ/(µl) | Total/(µl) |
|---|
| psb3c5(8/18miniprep) [1'], [2'], [3'] mixed / 50 | 1.0 | | | 1.0 | 5.6 | | | 56.6 |
8/19
Transformation
Li, Iguchi, Notsu
| Sample Name | Sample Volume (µl) | Competent Cells/(µl) | Medium |
|---|
| BBa_ J61002 p+ | 2 | DH5a /20 | LB(Amp) |
| BBa_J61002 p- | 2 | DH5a /20 | LB(Amp) |
| BBa_1A2 RBS | 2 | DH5a /20 | LB(Amp) |
Failed due to deactivation of Amp
8/19
MutationPCR
| Templates /µl | Primer /µl | Primer STAR Max Premix /µl | MilliQ /µl | Total(µl) |
|---|
| scfv plasmid /0.1 | m.scfv FW /1, m.scfvRV/1 | 25 | 22.9 | 50 |
| scfv plasmid (nega) /0.1 | 0 | 5 | 4.9 | 10 |
| (nega) /0 | m.scfv FW/0.2, m.scfvRV/0.2 | 5 | 4.6 | 10 |
---30cycle---
98℃ 10''
55℃ 15''
72℃ 20''
----------------
4℃ holding
8/19
Electrophoresis
Notsu
| Lane | sample/(µl) | Length(bp) |
|---|
| 1 | 1kb ladder/2 | - |
| 2 | scfv plasmid +primer(m.scfv FW, m.scfv RV) mutation PCR product /5 | 3600 |
| 3 | scfv plasmid mutation PCR product /5 | - |
| 4 | primer(m.scfv FW, m.scfv RV) mutation PCR product /5 | - |
| 5 | psb3c5 EPcut product /5 | 2600(backborn), 1200(inseert) |

[img22]
8/19
Concentration
Li, Notsu
| Sample | OD600 | Bacterial cells /ml |
|---|
| INP [1] K811005 | 0.643 | 5.14x10^8 |
| scfv06 K875006 | 0.634 | 5.07x10^8 |
| scfv04 K875004 | 0.618 | 4.94x10^8 |
1 OD 600 equals to 8x10^8 cells /ml
8/19
Miniprep
Li, Notsu
| DNA | Concentration/(µg/ml) | 260/280 | 260/230 |
|---|
| scfv 04[1] K875004 /3µl | 56 | 1.63 | 1.45 |
| scfv 04[2] K875004 /3µl | 56 | 1.63 | 1.45 |
| scfv 04[3] K875004 /3µl | 56 | 1.63 | 1.45 |
| scfv 06[1] K875006 /3µl | 56 | 1.63 | 1.45 |
| scfv 06[2] K875006 /3µl | 56 | 1.63 | 1.45 |
| scfv 06[3] K875006 /3µl | 56 | 1.63 | 1.45 |
| INP [1] K811005 /3µl | 56 | 1.63 | 1.45 |
| INP [2] K811005 /3µl | 56 | 1.63 | 1.45 |
| INP [3] K811005 /3µl | 56 | 1.63 | 1.45 |
There was a problem with an instrument
8/20
Transformation
Li, Uchino
| Sample Name | Sample Volume (µl) | Competent Cells/(µl) | Medium |
|---|
| 8/19 mutation PCR scfv plasmid (nega) | 1 | DH5a/ 20 | LB(CP) |
8/19 mutation PCR:
1µl extraction of scfv plasmid (nega)/10µl was folded 300 times.
8/20
Transformation
Li, Uchino
| Sample Name | Sample Volume (µl) | Competent Cells/(µl) | Medium |
|---|
| 8/19 mutation PCR scfv plasmid | 1 [note:1] | DH5a/20 | LB(AMP) |
| 8/19 mutation PCR scfv plasmid (nega) | 1 [note:2] | DH5a/20 | LB(AMP) |
| BBa_J61002 p+ | 1 | DH5a/20 | LB(AMP) |
| BBa_J61002 p- | 1 | DH5a/20 | LB(AMP) |
| BBa_1A2 RBS | 1 | DH5a/20 | LB(AMP) |
[note:1]
8/19 mutation PCR:
1µl extraction of scfv plasmid (nega)/50µl was folded 300 times.
[note:2]
8/19 mutation PCR:
1µl extraction of scfv plasmid (nega)/10µl was folded 300 times.
8/20
Electrophoresis
Li
| Lane | sample/(µl) | Length(bp) |
|---|
| 1 | 1kb ladder /1.5 | |
| 2 | 9/19 miniprep scfv04 [1] | |
| 3 | 9/19 miniprep scfv04 [2] | |
| 4 | 9/19 miniprep scfv04 [3] | |
| 5 | 9/19 miniprep scfv06 [1] | |
| 6 | 9/19 miniprep scfv06 [2] | |
| 7 | 9/19 miniprep scfv06 [3] | |
| 8 | 9/19 miniprep INP [1] | |
| 9 | 9/19 miniprep INP [2] | |
| 10 | 9/19 miniprep INP [3] | |

[img26]
8/21
Colony PCR
Uchino
| Colony | Primers |
|---|
| 8/20 transformation RBS | VF+VR |
| 8/20 transformation p+ | VF+VR |
| 8/20 transformation p- | VF+VR |
| 8/20 dilution INP | VF+VR |
8/21
PCR (Steps)
Uchino
| Steps | Temparature | Time | Cycle |
|---|
| Pre | 94℃ | 2min | 1 |
| De | 94℃ | 30 sec | 30 |
| An | 55℃ | 30 sec | 30 |
| Ex | 68℃ | 1min | 30 |
| Final E | 4℃ | 7min | 1 |
| Final Hold | 4℃ | - | - |
Autoclaved distrilled water /23µl
2xQuick taq /25µl
10 pmol/µl primer (VF) /1µl
10 pmol/µl primer (VR) /1µl
Template DNA /0µl
Total 50µl
8/21
Electrophoresis
Li
| Lane | sample/(µl) | Length(bp) |
|---|
| 1 | 1kb ladder /2 | |
| 2 | 8/21 colony PCR RBS /all | |
| 3 | 8/21 colony PCR p+ /all | |
| 4 | 8/21 colony PCR p- /all | |
| 5 | 8/21 colony PCR INP /all | |
| 6 | 8/21 colony PCR Nega /all | |


[img29-1]
weak band except INP -> retry
colony PCR and Electrophoresis were conducted under the same condition.
[img29-2]
RBS and INP success.
P+ and P- failed -> retry.
8/21
PCR (Steps)
Li
| Steps | Temparature | Time | Cycle |
|---|
| Predenature | 95℃ | 3min | 1 |
| Denature | 98℃ | 20sec | 25 |
| Annealing | 70℃ | 15sec | 25 |
| Extension | 72℃ | 30sec | 25 |
| Final Elongation | 72℃ | 5min | 1 |
| Final Holding | 4℃ | - | 1 |
8/21
PCR (Target)
Li
| Templates/(µl) | Primer1/(µl) | Primer2/(µl) | Buffer/(µl) | MilliQ/(µl) | Any Other/(µl) | Total/(µl) |
|---|
| scfv 8/20 transformation | cscfv.f /0.75 | cscfv.r /0.75 | 2x KAPA Hifi /12.5 | 11 | | 25 |
| - (nega) | cscfv.f /0.3 | cscfv.f /0.3 | 2x KAPA Hifi /5 | 4.4 | | 10 |
8/21
Colony PCR
Li
| Colony | Primers |
|---|
| 8/20 transformation p+ [1] | VF+VR |
| 8/20 transformation p+ [2] | VF+VR |
| 8/20 transformation p+ [3] | VF+VR |
| 8/20 transformation p- [1] | VF+VR |
| 8/20 transformation p- [2] | VF+VR |
| 8/20 transformation p- [3] | VF+VR |
| 8/18 parts p+ | VF+VR |
| 8/18 parts p- | VF+VR |
8/21
PCR (Steps)
Li
| Steps | Temparature | Time | Cycle |
|---|
| pre | 94℃ | 2min | 1 |
| de | 94℃ | 30sec | 30 |
| an | 55℃ | 30sec | 30 |
| ex | 68℃ | 1min | 30 |
| F.elong | 4℃ | 7min | 1 |
| F.hold | 4℃ | - | 1 |
8/21
Liquid Culture
Li
| Name | medium |
|---|
| strain | Medium/ml |
| 8/20 transformation RBS | PG(AMP) /6 |
| 8/20 dilution INP7 | PG(CP) /6 |
| 8/17 transformation 3c5, RFP | PG(CP) /6 |
| 8/20 transformation p+ [1] | PG(AMP) /6 |
| 8/20 transformation p- [1] | PG(AMP) /6 |
8/21
Electrophoresis
Li
| Lane | sample/(µl) | Length(bp) |
|---|
| 1 | 1kb ladder /1.5 | |
| 2 | 8/21 cloning PCR scfv[1] /2 | |
| 3 | 8/21 cloning PCR scfv[2] /2 | |
| 4 | nega /2 | |
| 5 | 8/21 colony PCR p+ [1] /2 | |
| 6 | 8/21 colony PCR p+ [2] /2 | |
| 7 | 8/21 colony PCR p+ [3] /2 | |
| 8 | 8/21 colony PCR p- [1] /2 | |
| 9 | 8/21 colony PCR p- [2] /2 | |
| 10 | 8/21 colony PCR p- [3] /2 | |
| 11 | 8/21 colony PCR parts p+ /2 | |
| 12 | 8/21 colony PCR parts p- /2 | |

[img35]
2, 3, 4 cloning PCR failed.
1 forgot to put ladder
5, 7, 8, 9 strong band
6 weak
10 failed
P+[1] and p-[1] -> liquid culture
8/24
Ligation
Li
| sample/µl | MilliQ (µl) | Buffer /µl | Ligase(µl) | Total(µl) |
|---|
| 8/19 mutation PCR scfv /5 | 3.5 | 1 | 0.5 | 10 |
8/24
Liquid Culture
Li
| Name | medium |
|---|
| 8/20 transformation m.scfv colonyA /6 | PG(AMP) |
8/24
Miniprep
Li
| DNA | Concentration/(µg/ml) | 260/280 | 260/230 |
|---|
| 8/20 Liquid culture RBS | 124.5 | 1.65 | 1.02 |
| 8/20 Liquid culture INP | 147.8 | 1.66 | 0.98 |
| 8/20 Liquid culture 3c5.RFP | 234.8 | 1.79 | 1.62 |
| 8/20 Liquid culture P+[1] | 40.3 | 1.78 | 1.60 |
| 8/20 Liquid culture P-[1] | 56.7 | 1.80 | 2.05 |
8/25
Miniprep
Li, Uchino, Notsu, Kaneko
| DNA | Concentration/(µg/ml) | 260/280 | 260/230 |
|---|
| 8/24 LIquid Culture m.scfv colonyA | - | - | - |
| 8/24 LIquid Culture m.scfv colonyB | - | - | - |
8/25
Electrophoresis
| Lane | sample/(µl) | Length(bp) |
|---|
| 1 | 1kb ladder /1.5 | |
| 2 | 8/24 miniprep p+(J61002) /2 | |
| 3 | 8/24 miniprep p-(J61002) /2 | |
| 4 | 8/24 miniprep RFP (3c5) /2 | |
| 5 | 8/24 miniprep INP (1c3) /2 | |
| 6 | 8/24 miniprep RBS (1A2) /2 | |

[img38]
8/25
Restriction Enzyme Digestion
Uchino
| Sample | DNA/(µl) | EcoR1/(µl) | Xba1/(µl) | Spe1/(µl) | Pst1/(µl) | Buffer/(µl) | BSA/(µl) | MilliQ/(µl) | Total/(µl) |
|---|
| p+/24.8 | | | 1 | 1 | 10xH /4 | | 9.2 | 40 |
| IINP /6.8 | | 1 | | 1 | 10xM /4 | | 27.2 | 40 |
| p- /17.6 | | | 1 | 1 | 10xH /4 | | 16.4 | 40 |
| RBS /8.0 | | | 1 | 1 | 10xH /4 | | 26 | 40 |
| RFP /4.3 | 1 | | | 1 | 10xH /4 | | 29.7 | 40 |
8/25
Gel Extraction
Uchino, Notsu
| Lane | Restriction Enzyme Digestion Product | Volume/(µl) |
|---|
| 1 | 1kb ladder | 6 |
| 2, 3 | 8/25 digestion p+ | 24 each |
| 4, 5 | 8/25 digestion p- | 24 each |
| 6, 7 | 8/25 digestion RFP | 24 each |
| 8, 9 | 8/25 digestion INP | 24 each |
| 10, 11 | 8/25 digestion RBS | 24 each |

[img40]
8/25
Actvation check of Xba and Pst
Uchino
| INP(µl) | Xba(µl) | Pst(µl) | 10xM(µl) | BSA(µl) | MilliQ(µl) | Total(µl) |
|---|
| 0.68 | 0.1 | 0.1 | 1 | 0.2 | 7.92 | 10 |
| 0.68 | 0.1 | - | 1 | 0.2 | 8.02 | 10 |
| 0.68 | - | 0.1 | 1 | 0.2 | 8.02 | 10 |
| 0.68 | - | - | 1 | 0.2 | 8.12 | 10 |
8/25
Electrophoresis
Notsu
| Lane | sample/(µl) | Length(bp) |
|---|
| 1 | 1kb ladder /3 | |
| 2 | [1] (inp xp cut) /10 | |
| 3 | [2] (inp x cut) /10 | |
| 4 | [3] (inp p cut) /10 | |
| 5 | [4] (inp no cut) /10 | |

[img42]
8/26
Restriction Enzyme Digestion
Uchino, Notsu
| Sample | DNA/(µl) | EcoR1/(µl) | Xba1/(µl) | Spe1/(µl) | Pst1/(µl) | Buffer/(µl) | BSA/(µl) | MilliQ/(µl) | Total/(µl) |
|---|
| 8/24 miniprep INP 1c3 /0.68 | - | 1.0 | | | | 0.2 | 7.12 | 10 |
| 8/15 miniprep RFP psb1c3 /1.3 | - | 1.0 | | | | 0.2 | 6.5 | 10 |
| 8/24 miniprep INP 1c3 /0.68 | 0.1 | 1.0 | | | | 0.2 | 7.02 | 10 |
| 8/15 miniprep RFP psb1c3 /1.3 | 0.1 | 1.0 | | | | 0.2 | 6.4 | 10 |
| 8/24 miniprep INP 1c3/ 0.68 | 0.1 | - | | | | 0.2 | 8.02 | 10 |
| 8/15 miniprep RFP psb1c3 /1.3 | 0.1 | - | | | | 0.2 | 7.4 | 10 |
| 8/24 miniprep INP 1c3 /0.68 | - | - | | | | 0.2 | 8.12 | 10 |
| 8/15 miniprep RFP psb1c3 /1.3 | - | - | | | | 0.2 | 7.5 | 10 |
8/26
PCR (Target)
Li
| Templates/(µl) | Primer1/(µl) | Primer2/(µl) | Buffer/(µl) | MilliQ/(µl) | Any Other/(µl) | Total/(µl) |
|---|
| scfv from MK | c.scfv fw/0.75 | c.scfv rv/0.75 | 2x KAPA Hifi /12.5 | 11 | | 25 |
| none | c.scfv fw/0.75 | c.scfv rv/0.75 | 2x KAPA Hifi /5 | 4.4 | | 10 |
8/26
Activation Check of Xba
Uchino
| Lane | sample/(µl) | Length(bp) |
|---|
| 1 | 1kb ladder/2 | |
| 2 | [8] RFP/10 | |
| 3 | [6] RFP EcoRI/10 | |
| 4 | [2] RFP XbaI/10 | |
| 5 | [4] RFP EcoRI, XbaI /10 | |
| 6 | [7] INP /10 | |
| 7 | [5] INP EcoRI /10 | |
| 8 | [1] INP XbaI /10 | |
| 9 | [3] INP EcoRI, XbaI/10 | |

[img45-1]
[img45-2]
8/27
Electrophoresis
Li
| Lane | sample/(µl) | Length(bp) |
|---|
| 2 | 1kb ladder | 2 |
| 3 | 8/25 PCR scfv | 5 |
| 4 | 8/25 PCR none | 5 |

8/27
PCR (Target)
Li
| Templates/(µl) | Primer1/(µl) | Primer2/(µl) | Buffer/(µl) | MilliQ/(µl) | Any Other/(µl) | Total/(µl) |
|---|
| s.BAN | BAN.Fw/ 0.75 | BAN.Rv/ 0.75 | | 11 | 2xKAPA HiFi/ 12.5 | 25 |
| s.BAN | BAN.Fw/ 0.75 | BAN.Rv/ 0.75 | | 11 | 2xKAPA HiFi/ 12.5 | 25 |
| s.BAN | BAN.Fw/ 0.75 | BAN.Histag/ 0.75 | | 11 | 2xKAPA HiFi/ 12.5 | 25 |
| scfv | scfv.Fw/ 0.75 | scfv.Rv/ 0.75 | | 11 | 2xKAPA HiFi/ 12.5 | 25 |
| 8/24 miniprep INP | INP.Fw/ 0.75 | INP.Rv/ 0.75 | | 11 | 2xKAPA HiFi/ 12.5 | 25 |
| 8/24 miniprep INP | INP.Fw/ 0.75 | INP.Rv.Histag/ 0.75 | | 11 | 2xKAPA HiFi/ 12.5 | 25 |
8/27
PCR (Steps)
Li
| Steps | Temparature | Time | Cycle |
|---|
| Predenature | 95 | 3min | 1 |
| Denature | 98 | 20sec | 25 |
| Annealing | 65 | 15sec | 25 |
| Extension | 72 | 30sec | 25 |
| Final Elogation | 72 | 5min | 1 |
| Final Holding | 4 | - | 1 |
9/8
Restriction Enzyme Digestion
Notsu
| Sample | DNA/(µl) | EcoR1/(µl) | Xba1/(µl) | Spe1/(µl) | Pst1/(µl) | Buffer/(µl) | BSA/(µl) | MilliQ/(µl) | Total/(µl) |
|---|
| [1] 8/15 miniprep 1c3 RFP /14 | 0.5 | | 0.5 | - | 10xH /3 | | 12 | 30 |
| [2] 8/24 miniprep 3c5 RFP /8.5 | 0.5 | | - | 0.5 | 10xH /3 | | 17.5 | 30 |
| [3] 8/24 miniprep 3c5 RFP /8.5 | 0.5 | | 0.5 | - | 10xH /3 | | 17.5 | 30 |
| [4] 8/24 miniprep BBa_J61002 p- /24 | 0.5 | | - | 0.5 | 10xH /3 | | 2 | 30 |

9/9
Electrophoresis
Notsu
| Lane | Restriction Enzyme Digestion Products |
|---|
| 1 | 100bp ladder 9µL |
| 2 | 8/24 miniprep p- EPcut 30µL |
9/9
Electrophoresis
Sukegawa, Notsu
| Lane | Restriction Enzyme Digestion Products |
|---|
| 1 | 1kbp ladder 9µL |
| 2 | 8/15 miniprep 1C3RFP EScut 30µL |
| 3 | 8/24 miniprep 3C5RFP EPcut 30µL |
| 4 | 8/24 miniprep 3C5RFP EScut 30µL |
9/14
PCR (Target)
Li
| Templates/(µl) | Primer1/(µl) | Primer2/(µl) | Buffer/(µl) | MilliQ/(µl) | Any Other/(µl) | Total/(µl) |
|---|
| 8/24 miniprep INP | INPfw/0.75 | INPRv/0.75 | 2×KAPAHiFi | 11 | | 25 |
| 8/24 miniprep INP | INPfw/0.75 | INPHisRv/0.75 | 2×KAPAHiFi | 11 | | 25 |
| BAN | BANFw/0/75 | BANRv/0.75 | 2×KAPAHiFi | 11 | | 25 |
| BAN | BANFw/0/75 | BANHisRv/0.75 | 2×KAPAHiFi | 11 | | 25 |
| BAN | BANFw/0/75 | RBSRv/0.75 | 2×KAPAHiFi | 11 | | 25 |
| scFv fromMk | scFvFw/0/75 | scFvRv/0/75 | 2×KAPAHiFi | 11 | | 25 |
| scFv fromMk | scFvHisFw/0.75 | scFvRv/0.75 | 2×KAPAHiFi | 11 | | 25 |
9/14
PCR (Steps)
Li
| Steps | Temparature | Time | Cycle |
|---|
| 1 | 95 | 3min | 1 |
| 2 | 98 | 70sec | 25 |
| 3 | 68 | 15sec | 25 |
| 4 | 72 | 30sec | 25 |
| 5 | 72 | 5min | 1 |
| 6 | 10 | - | 1 |
9/14
Gel Extraction
Kaneko
| Lane | Restriction Enzyme Digestion Products |
|---|
| 2 | ladder/6 |
| 4 | 9/14 PCR1/10 |
| 6 | 9/14 PCR2/10 |
| 8 | 9/14 PCR3/10 |
| 10 | 9/14 PCR4/10 |
| 12 | 9/14PCR5/10 |
| 14 | 9/14 PCR6/10 |
| 16 | 9/14 PCR7/10 |


9/14
PCR (Target)
Li
| Templates/(µl) | Primer1/(µl) | Primer2/(µl) | Buffer/(µl) | MilliQ/(µl) | Any Other/(µl) | Total/(µl) |
|---|
| 3sBAN/0.15+6scFvfromMK/0.15 | BANFw/0/75 | scFvRv/0.75 | 2×KAPAHiFi/12.5 | | | 25 |
| 4sBAN/0.15+7scFvfromMK/0.15 | BANFw/0/75 | scFvRv/0.75 | 2×KAPAHiFi/12.5 | | | 25 |
| 1INP8/24miniprep/0.15+5sBAN0.15+scFvfromMK/0.15 | BANFw/0/75 | scFvRv/0.75 | 2×KAPAHiFi/12.5 | | | 25 |
| 2INP8/24miniprep/0.15 | BANFw/0/75 | scFvRv/0.75 | 2×KAPAHiFi/12.5 | | | 25 |
9/15
Restriction Enzyme Digestion
Li, Uchino
| Sample | DNA/(µl) | EcoR1/(µl) | Xba1/(µl) | Spe1/(µl) | Pst1/(µl) | Buffer/(µl) | BSA/(µl) | MilliQ/(µl) | Total/(µl) |
|---|
| 3sBAN+6scFvfromMKPCRproduct | 34 | 1 | | 1 | | 4 | | | 40 |
| 4sBAN+7scFvfromMK PCRproduct | 34 | 1 | | 1 | | 4 | | | 40 |
| 1INP8/24miniprep+5sBAN+6scFvfromMKPCRproduct | 16 | 1 | | 1 | | 2 | | | 20 |
| 2INP8/24miniprep+5sBAN+7scFvfromMkPCRproduct | 34 | 1 | | 1 | | 4 | | | 40 |
9/15
Gel Extraction
Li, Uchino
| Lane | Restriction Enzyme Digestion Product | Volume/(µl) |
|---|
| 1 | 1kb ladder | |
| 2 | 3sBAN+6scFvfromMkPCRproductEScut | 20 |
| 3 | 3sBAN+6scFvfromMkPCRproductEScut | 20 |
| 4 | 4sBAN+7scFvfromMkPCRproductEScut | 20 |
| 5 | 4sBAN+7scFvfromMkPCRproductEScut | 20 |
| 6 | 1INP8/24miniprep+5sBAN+7scFvfromMkPCRproductEScut | 20 |
| 7 | 2INP8/24miniprep+5sBAN+7scFvfromMkPCRproductEScut | 20 |
| 8 | 2INP8/24miniprep+5sBAN+7scFvfromMkPCRproductEScut | 20 |
| 9 | RFP3C5PCRproduct | |


9/16
Colony PCR (2Column)
Uchino, Michimori
| Name I | Name II |
|---|
| 9/15 transformation BANscFv 3C5 colony1~16 | VF+VR |
| 9/15 transformation INPscFv 3C5 colony1~16 | VF+VR |
| 9/15 transformation BANHisscFv 3C5 colony1~16 | VF+VR |
| 9/15 transformation INPHisscFv 3C5 colony1~16 | VF+VR |
9/16
PCR (Steps)
Uchino, Michimori
| Steps | Temparature | Time | Cycle |
|---|
| 1 | 94 | 2 | 1 |
| 2 | 94 | 0.5 | 25 |
| 3 | 55 | 0.5 | 25 |
| 4 | 68 | 1 | 25 |
| 5 | 4 | 7 | 1 |
| 6 | 4 | - | 1 |
9/16
Gel Extraction
Li, Kaneko, Notsu
| Lane | Restriction Enzyme Digestion Product | Volume/(µl) |
|---|
| 1 | 1kb ladder | 5 |
| 2 | BANscFv3C5 colony 9 | 10 |
| 3 | BANscFv3C5 colony 10 | 10 |
| 4 | BANscFv3C5 colony 11 | 10 |
| 5 | BANscFv3C5 colony 12 | 10 |
| 6 | BANscFv3C5 colony 13 | 10 |
| 7 | BANscFv3C5 colony 14 | 10 |
| 8 | BANscFv3C5 colony 15 | 10 |
| 9 | BANscFv3C5 colony 16 | 10 |
| 10 | BANHisscFv3C5 colony 9 | 10 |
| 11 | BANHisscFv3C5 colony 10 | 10 |
| 12 | BANHisscFv3C5 colony 11 | 10 |
| 13 | BANHisscFv3C5 colony 12 | 10 |
| 14 | BANHisscFv3C5 colony 13 | 10 |
| 15 | BANHisscFv3C5 colony 14 | 10 |
| 16 | BANHisscFv3C5 colony 15 | 10 |
| 17 | BANHisscFv3C5 colony 16 | 10 |
| 18 | BANscFv3C5 colony 1 | 10 |
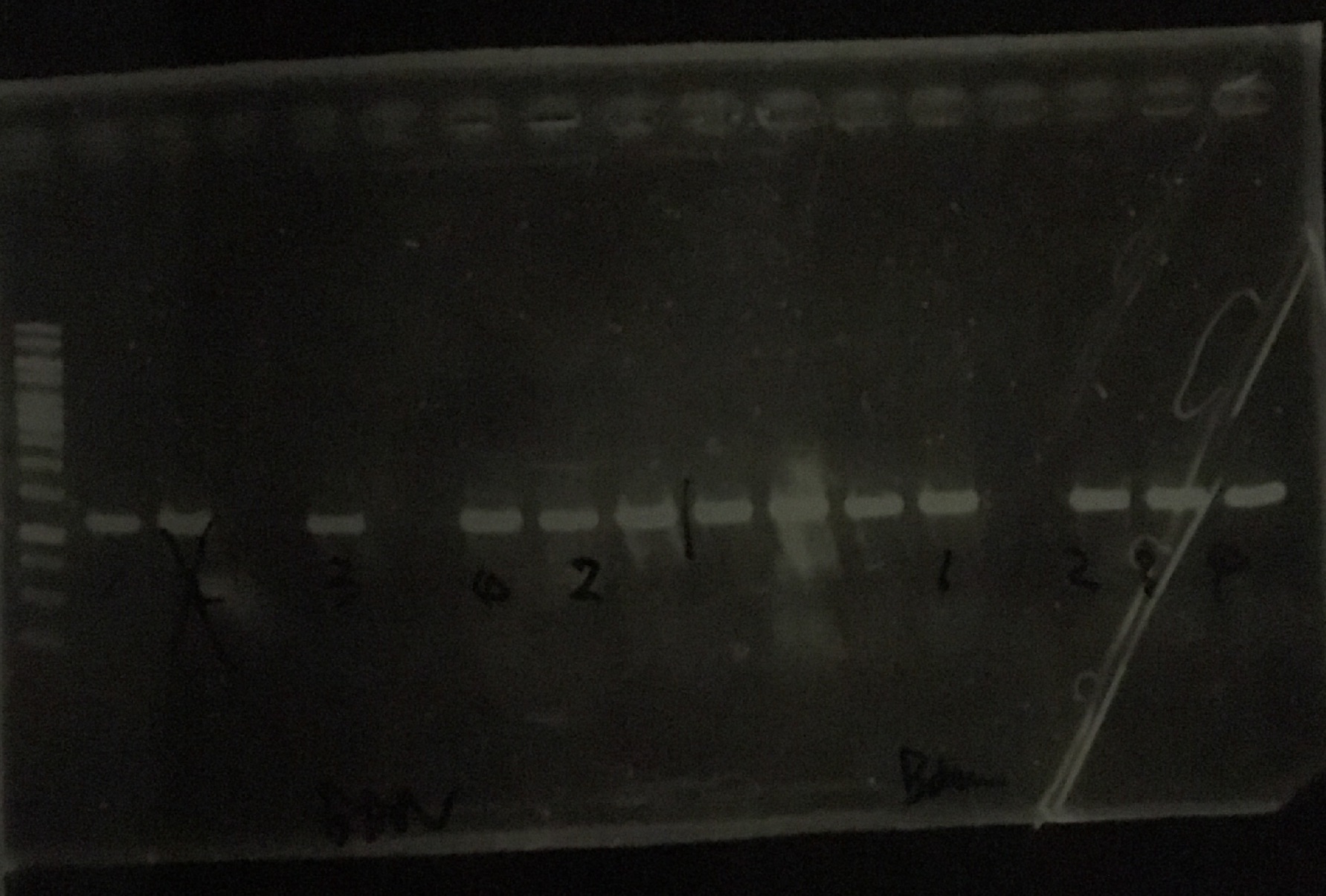



9/16
Gel Extraction
Li, Kaneko, Notsu
| Lane | Restriction Enzyme Digestion Product | Volume/(µl) |
|---|
| 1 | 1kb ladder | 5 |
| 2 | INPscFv3C5 colony 9 | 10 |
| 3 | INPscFv3C5 colony 10 | 10 |
| 4 | INPscFv3C5 colony 11 | 10 |
| 5 | INPscFv3C5 colony 12 | 10 |
| 6 | INPscFv3C5 colony 13 | 10 |
| 7 | INPscFv3C5 colony 14 | 10 |
| 8 | INPscFv3C5 colony 15 | 10 |
| 9 | INPscFv3C5 colony 16 | 10 |
| 10 | INPHisscFv3C5 colony 9 | 10 |
| 11 | INPHisscFv3C5 colony 10 | 10 |
| 12 | INPHisscFv3C5 colony 11 | 10 |
| 13 | INPHisscFv3C5 colony 12 | 10 |
| 14 | INPHisscFv3C5 colony 13 | 10 |
| 15 | INPHisscFv3C5 colony 14 | 10 |
| 16 | INPHisscFv3C5 colony 15 | 10 |
| 17 | INPHisscFv3C5 colony 16 | 10 |
| 18 | BANscFv3C5 colony 1 | 10 |


![]()


9/17
Electrophoresis
Michimori, Kaneko
| Lane | Restriction Enzyme Digestion Products |
|---|
| 1 | buffer/5 |
| 2 | 1kb ladder/5 |
| 3 | BAN 9 P/4.5 |
| 4 | BAN 9 N/4.5 |
| 5 | BAN14 P/4.5 |
| 6 | BAN14 N/4.5 |
| 7 | BANHis14 P/4.5 |
| 8 | BANHis14/4.5 |

9/17
Electrophoresis
Kaneko
| Lane | sample/(µl) | Length(bp) |
|---|
| 1 | 1kbp ladder | |
| 2 | none | |
| 3 | BANHis16 p | |
| 4 | BANHis16 n | |
| 5 | INP 12 p | |
| 6 | INP 14 p | |
| 7 | INP 12 n | |
| 8 | INP 14 n | |

9/17
Electrophoresis
Kaneko
| Lane | Restriction Enzyme Digestion Products |
|---|
| 1 | - |
| 2 | - |
| 3 | 1kbladder/5 |
| 4 | INPHis9 P/4.5 |
| 5 | INPHis9N/4.5 |
| 6 | INPHis12P/4.5 |
| 7 | INPHis12N/4.5 |
| 8 | INP14 N/4.5 |

9/17
Restriction Enzyme Digestion
Uchino, Michimori
| Sample | DNA/(µl) | EcoR1/(µl) | Xba1/(µl) | Spe1/(µl) | Pst1/(µl) | Buffer/(µl) | BSA/(µl) | MilliQ/(µl) | Total/(µl) |
|---|
| BAN9 | 2 | 0.5 | | | 0.5 | 10×H/1 | | 6 | 10 |
| BAN9 | 1 | 0 | | | 0 | 10×H/1 | | 8 | 10 |
| BAN14 | 2 | 0.5 | | | 0.5 | 10×H/1 | | 6 | 10 |
| BAN14 | 1 | 0 | | | 0 | 10×H/1 | | 8 | 10 |
| BANHis14 | 2 | 0.5 | | | 0.5 | 10×H/1 | | 6 | 10 |
| BANHis 14 | 1 | 0 | | | 0 | 10×H/1 | | 8 | 10 |
| BANHis 16 | 2 | 0.5 | | | 0.5 | 10×H/1 | | 6 | 10 |
| BANHis16 | 1 | 0 | | | 0 | 10×H/1 | | 8 | 10 |
| INP12 | 2 | 0.5 | | | 0.5 | 10×H/1 | | 6 | 10 |
| INP12 | 1 | 0 | | | 0 | 10×H/1 | | 8 | 10 |
| INP14 | 2 | 0.5 | | | 0.5 | 10×H/1 | | 6 | 10 |
| INP 14 | 1 | 0 | | | 0 | 10×H/1 | | 8 | 10 |
| INP 9 | 2 | 0.5 | | | 0.5 | 10×H/1 | | 6 | 10 |
| INP9 | 1 | 0 | | | 0 | 10×H/1 | | 8 | 10 |
| INPHis12 | 2 | 0.5 | | | 0.5 | 10×H/1 | | 6 | 10 |
| INPHis12 | 1 | 0 | | | 0 | 10×H/1 | | 8 | 10 |

9/17
Miniprep
Notsu
| DNA | Concentration/(µg/ml) | 260/280 | 260/230 |
|---|
| 9/16 liquid culture BANscFv12 | | | |
| 9/16 liquid culture BANscFv14 | | | |
| 9/16 liquid culture BANHisscFv12 | | | |
| 9/16 liquid culture BANHisscFv14 | | | |
| 9/16 liquid culture INPHisscFv12 | | | |
| 9/16 liquid culture INPHisscFv14 | | | |
| 9/16 liquid culture INPHisscFv10 | | | |
| 9/16 liquid culture BANscFv | | | |








































