| Line 1: | Line 1: | ||
| + | |||
<html> | <html> | ||
| − | + | <style> | |
| + | #content{width:100%;padding:0px;margin:0px;background-color: #ededed;} | ||
| + | #sideMenu, #top_title | ||
| + | { | ||
| + | display: none; | ||
| + | } | ||
| + | body{font: 16px sans-serif;} | ||
| + | .footer_bg .footer { | ||
| + | padding: 0; | ||
| + | border-top: 6px solid #222222; | ||
| + | } | ||
| + | .footer_bg .footer_btm { | ||
| + | margin-top: 0; | ||
| + | border-top: none; | ||
| + | padding: 2% 4%; | ||
| + | padding-top: 2%; | ||
| + | max-width: 1458px; | ||
| + | margin: 0 auto; | ||
| + | } | ||
| + | </style> | ||
<head> | <head> | ||
| − | + | <meta charset="UTF-8"> | |
| − | + | <title></title> | |
| − | + | <link rel="stylesheet" href="https://2016.igem.org/Team:TJUSLS_China/home/index_style?action=raw&ctype=text/css"> | |
| − | + | <script type="text/javascript" src="https://2016.igem.org/Team:TJUSLS_China/home/index_js_two?action=raw&ctype=text/javascript"></script> | |
| − | + | <script type="text/javascript" src="https://2016.igem.org/Team:TJUSLS_China/home/index_js_one?action=raw&ctype=text/javascript"></script> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | action=raw&ctype=text/javascript"></script> | + | |
</head> | </head> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<body> | <body> | ||
| − | <div class=" | + | <!--menu--> |
| − | + | <div class="header-box"> | |
| − | + | <div class="header"> | |
| − | + | <!-- start h_menu4 --> | |
| + | <div class="h_menu4"> | ||
| + | <a class="toggleMenu" href="">Menu</a> | ||
| + | <ul id="top_ul_my" class="nav"> | ||
| + | <script> | ||
| + | function getHover(number){ | ||
| + | var top_ul_my_lis = $("#top_ul_my").find(">li"); | ||
| + | console.log(top_ul_my_lis); | ||
| + | if(number == 0) top_ul_my_lis[0].setAttribute("class", "active hover"); | ||
| + | else top_ul_my_lis[0].setAttribute("class", "active"); | ||
| − | + | for(var i = 1; i < top_ul_my_lis.length; i++){ | |
| − | + | if(i == number) { | |
| − | + | top_ul_my_lis[i].setAttribute("class", "hover"); | |
| − | + | }else{ | |
| − | + | top_ul_my_lis[i].setAttribute("class", ""); | |
| − | + | } | |
| − | + | } | |
| − | + | } | |
| − | + | $(".header").hover(function(event){ | |
| + | event.stopPropagation(); | ||
| + | $('#top_ul_my li').attr("class",""); | ||
| + | }); | ||
| − | + | </script> | |
| − | + | <li onmouseover="getHover(0)"><a href="https://2016.igem.org/Team:TJUSLS_China/home">TJUSLS</a></li> | |
| − | + | <li class="active" onmouseover="getHover(1)" class=""><a class="active" href="https://2016.igem.org/Team:TJUSLS_China/Project" class="root">project</a> | |
| − | + | <ul> | |
| − | + | <li><a href="https://2016.igem.org/Team:TJUSLS_China/Description">Description</a></li> | |
| − | + | <li><a href="https://2016.igem.org/Team:TJUSLS_China/Design">Design</a></li> | |
| − | + | <li><a href="https://2016.igem.org/Team:TJUSLS_China/Experiments">Experiments</a></li> | |
| − | + | <li><a href="https://2016.igem.org/Team:TJUSLS_China/Proof">Proof of Concept</a></li> | |
| + | <li><a href="https://2016.igem.org/Team:TJUSLS_China/Demonstrate">Demonstrate</a></li> | ||
| − | + | <li><a href="https://2016.igem.org/Team:TJUSLS_China/Notebook">Notebook</a></li> | |
| − | + | <li><a href="https://2016.igem.org/Team:TJUSLS_China/PROJECT/PROTOCOLS">Protocols</a></li> | |
| − | + | </ul> | |
| − | + | </li> | |
| − | + | <li onmouseover="getHover(2)" class=""><a href="https://2016.igem.org/Team:TJUSLS_China/Parts">PARTS</a> | |
| − | + | <ul> | |
| − | + | <li><a href="https://2016.igem.org/Team:TJUSLS_China/Basic_Part">Basic Parts</a></li> | |
| + | <li><a href="https://2016.igem.org/Team:TJUSLS_China/Composite_Part">Composite Parts</a></li> | ||
| + | <li><a href="https://2016.igem.org/Team:TJUSLS_China/Part_Collection">Part Collection</a></li> | ||
| − | + | </ul> | |
| − | + | </li> | |
| − | + | <li onmouseover="getHover(3)" class=""><a href="https://2016.igem.org/Team:TJUSLS_China/Safety">SAFETY</a></li> | |
| − | + | <li onmouseover="getHover(4)" class=""><a href="https://2016.igem.org/Team:TFJUSLS_China/Attributions">ATTRIBUTIONS</a> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | </li> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | <li onmouseover="getHover(5)" class=""><a href="https://2016.igem.org/Team:TJUSLS_China/Human_Practices">HUMAN PRACTICES</a> | |
| − | + | <ul> | |
| − | + | <li><a href="https://2016.igem.org/Team:TJUSLS_China/HP/Silver">Silver</a></li> | |
| − | + | <li><a href="https://2016.igem.org/Team:TJUSLS_China/HP/Gold">Gold</a></li> | |
| − | + | <li><a href="https://2016.igem.org/Team:TJUSLS_China/Integrated_Practices">Integrated Practices</a></li> | |
| − | + | <li><a href="https://2016.igem.org/Team:TJUSLS_China/Engagement">Engagement</a></li> | |
| + | </ul> | ||
| + | </li> | ||
| + | <li onmouseover="getHover(6)" class=""><a href="https://2016.igem.org/Team:TJUSLS_China/Modeling">MODELING</a> | ||
| + | <ul> | ||
| + | <li><a href="https://2016.igem.org/Team:TJUSLS_China/Modeling">Modeling</a></li> | ||
| + | <li><a href="https://2016.igem.org/Team:TJUSLS_China/Software">Software</a></li> | ||
| + | </ul> | ||
| + | </li> | ||
| + | <li onmouseover="getHover(7)" class=""><a href="https://2016.igem.org/Team:TJUSLS_China/Team">TEAM</a> | ||
| − | + | <ul> | |
| − | + | <li><a href="https://2016.igem.org/Team:TJUSLS_China/Team">Team</a></li> | |
| − | + | <li><a href="https://2016.igem.org/Team:TJUSLS_China/Collaborations">Collaborations</a></li> | |
| − | + | </ul> | |
| − | + | </li> | |
| − | + | </ul> | |
| − | + | <!--<script type="text/javascript" src="https://2016.igem.org/Team:TJUSLS_China/home/home_nav?action=raw&ctype=text/javascript"></script>--> | |
| − | + | </div> | |
| − | + | <!-- end h_menu4 --> | |
| − | + | <div class="clear"></div> | |
| − | + | </div> | |
| − | + | <div class="sec-menu"> | |
| − | + | <img class="sec-menu-dot" src="https://static.igem.org/mediawiki/2016/f/f9/T--TJUSLS_China--sec-menu.png"/> | |
| − | + | <img class="sec-menu-close" src="https://static.igem.org/mediawiki/2016/b/b5/T--TJUSLS_China--sec-menu-close.png"/> | |
| − | + | <ul> | |
| − | + | <li><a href="#part1">Overview</a></li> | |
| − | + | <li><a href="#part2">Application</a></li> | |
| − | + | <li><a href="#part3">Background</a></li> | |
| − | + | <li><a href="#part4">Structure and mutation</a></li> | |
| − | + | <li><a href="#part3">Surface Display</a></li> | |
| − | + | <li><a href="#part3">Fusing secretion of hydrophobin and PETase and their co - display</a></li> | |
| − | + | </ul> | |
| − | + | </div> | |
| − | + | </div> | |
| − | + | <!--title--> | |
| − | + | <div class="second-title"> | |
| − | + | Description | |
| − | + | <div class="second-title-img"> | |
| − | + | <img src="https://static.igem.org/mediawiki/2016/8/86/T--TJUSLS_China--second-logo.png"/> | |
| − | + | </div> | |
| − | + | </div> | |
| − | + | <!--content--> | |
| − | + | <div class="second-content"> | |
| − | + | <div class="sec-list-wenzi"> | |
| − | + | <h3 id="part1">Overview</h3> | |
| − | + | <div class="sec-wenzi-content">We put forward a new model about enzymology called Training Protein. It can be applied on every enzyme. According to its self-characteristic, people will do targeted modify on the enzyme in order to an extensive application.</div> | |
| − | + | <div class="sec-wenzi-content">Frist and the foremost, researchers should analyze the protein tertiary structure and understand its characteristic for well-directed proceeding to the next section. The next step has two paths, one is directed mutation, which site is chosen by analyzing the protein tertiary structure to find its functional domains, and the other is surface display. Surface display can improve the catalytic efficiency of the enzyme, and avoid the trouble of protein purification.</div> | |
| − | + | <div class="clear"></div> | |
| − | + | </div> | |
| − | + | <div class="sec-list-wenzi"> | |
| − | + | <h3 id="part2">Application</h3> | |
| − | + | <div class="sec-wenzi-content">TJUSLS China's subject of the competition for this year is to modify PET hydrolase (PETase) and developing cell surface display. PETase was found from a kind of microorganism living on PET as the main carbon source. It can degrade macromolecular polymers into monomers. Surface display can reveal the protein whose gene code is coalescing the gene code of target protein or polypeptide with the counterpart of ankyrin on the surface of the host cell wall to harvest the whole cell catalyst. The protagonists of our project, which are PETase and the surface display technology, will act in three following aspects.</div> | |
| − | + | <div class="sec-wenzi-content">Firstly, we find the enzyme catalytic center and its binding center by analyzing the structure of PETase with Protein crystallography and X ray diffraction technique, as well as choosing the mutation site under its character direction in order to carry out the directional mutation to improve the degradation efficiency and thermal stability. Moreover, using the prokaryotic (E. coli) and eukaryotic (Pichia Pastoris) surface display for whole cell catalysis. Thirdly, fusing the PETase and hydrophobic protein then expressing the fusion protein in Pichia Pastoris, which will take advantage of hydrophobic protein in hydrophobicity to give a hydrophobic environment for a better degradation efficiency. Meanwhile, the co-display combining PETase and hydrophobic protein in Pichia Pastoris will change the character of cells' surfaces so that cells can adapt the extreme environment, then the whole-cell biocatalyst might have higher catalytic efficiency as well as break the limitation set by reaction condition. According to that, the PETase degradation reaction conditions will be broaden which means it can be applied in industry easier.</div> | |
| − | + | <div class="clear"></div> | |
| − | + | </div> | |
| − | + | <div class="sec-list-wenzi"> | |
| − | + | <h3 id="part3">Background</h3> | |
| − | + | <div class="sec-wenzi-content">Polyethyleneterephthalate (PET) is the condensation of terephthalic acid and ethylene glycol. | |
| − | + | PET is mainly used as fiber in textile industry, sometime applying in film and engineering plastic. PET film can be used as electrical insulating material like capacitor, cable insulation, printed wiring substrate, electrode slot insulation, etc. The second application is base or baseband, as film, X-ray, tape, computer tape, etc. PET film can change into metallic films by vacuum aluminizing, like gold and silver wire, micro capacitor film, etc. The third direction is blow-molding products, for example tensile polyester bottle using in packing.</div> | |
| − | + | <div class="sec-wenzi-content">With the increasing demand of the society, because of its good fastness weather, electrical insulation, and having the friction resistance, high strength, good transparency, non-toxic, anti penetration, light quality, high production efficiency and some others advantages, the rapid growth of PET production and sales is consequent, which leads to the result that more and more obsolete PET has already caused the environment problem.</div> | |
| − | + | <div class="sec-wenzi-content">According to the report from Research and Markets, the PET global market will shows annual growth of 7.3 percent during 2014 to 2019. Its market value is expected to reach about 47 billion 400 million dollars until 2019. However, the obsolete PET, which will take up a lot of space and cause white pollution, has strong chemical inertia and difficult in degradation. In a word, PET degradation is the most urgent problem. </div> | |
| − | + | <div class="sec-wenzi-content">The mainly methods are landfill, burning and recycle. Landfill and burning are too simple to protect the environment. So degradation and recycling will be a better choice, like physical recycling, chemical degradation and biodegradation. </div> | |
| − | + | <div class="sec-wenzi-content">The physical recycling is a plastic reprocessing and recycling technology by cutting, smashing, Heating and melting. Although the physical way is low cost, it will cause the regeneration product performance delays with impurities accumulate. So it can only be degrade in using. </div> | |
| − | + | <div class="sec-wenzi-content">PET is fully dense and high crystallinity with ester bond in molecular chain, which is possible degraded. Under the high temperature, PET reacts with chemical reagents, called depolymerizing reaction, and turns into smaller molecule, intermediate raw material or units. Products can reuse as the crude material of PET after separation and purification. Even if chemical degradation realize the recycling of raw materials, it might cause equipment corrosion seriously as well as increasing energy and reagent consumption, which is hardly mean eco-friendly. </div> | |
| − | + | <div class="sec-wenzi-content">In the other words, biodegradation, which means taking advantages of microorganism, such as bacteria and fungus, to degraded plastic, has broad application prospects result from its low cost and high degradation efficiency. </div> | |
| − | + | <div class="clear"></div> | |
| − | + | </div> | |
| − | + | <div class="sec-list-wenzi"> | |
| − | + | <h3 id="part4">Structure and mutation</h3> | |
| − | + | <div class="h4-title">Background</div> | |
| − | + | <div class="sec-wenzi-content">PETase is the only enzyme found in bacteria which can degrade PET. Compare to the other enzyme found in fungi like LCC, TfH, FsC, PETase is much more active under low temperature environment (20~40℃), which means its reaction conditions is feasible in practical application than the others'. Besides,PETase is good at degrading highly-crystallized PET (such as plastic bottles) while others don’t, their enzyme activity to highly-crystallized PET is more than 20 times lower than PETase.However, we should not deny that the degradation rate of PETase is not enough for put on stream because of its activity. To get over this barrier, we manage to enhance its activity by site-direxted mutagenesis. The tertiary structure of a protein is closely coupled to its function. The tertiary structure of a protein is experimentally determined, predominantly by two methods called X-ray Diffraction and Protein Crystallography. According to that, we find the catalytic center and combination center of the enzyme, which will assist us to know how the enzyme works, and choose mutation sites on the basis of that.</div> | |
| − | + | <div class="tuwen-img-box"> | |
| − | + | <img src="https://static.igem.org/mediawiki/igem.org/4/43/Description25.jpg"/> | |
| − | + | <div>Compare to the other enzyme found in fungi like LCC, TfH, FsC, PETase is much more active under low temperature environment (20~40℃)</div> | |
| − | + | </div> | |
| − | + | <div class="references"> | |
| − | + | <div>References:</div> | |
| − | + | <div>[1] Yoshida S, Hiraga K, Takehana T, et al. A bacterium that degrades and assimilates poly(ethylene terephthalate).[J]. Science, 2016, 351(6278):1196-1199.</div> | |
| − | + | <div>[2] Kitadokoro, Kengo, Thumarat et al.(2012). Crystal structure of cutinase Est119 from Thermobifida alba AHK119 that can degrade modified polyethylene terephthalate at 1.76Å resolution. Polymer Degradation and Stability, 97(5), 771-775. doi: 10.1016/j.polymdegradstab.2012.02.003</div> | |
| − | + | <div>[3] Roth, C., Wei, R., Oeser, T. et al. (2014). Structural and functional studies on a thermostable polyethylene terephthalate degrading hydrolase from Thermobifida fusca. Appl Microbiol Biotechnol, 98(18), 7815-7823. doi: 10.1007/s00253-014-5672-0 </div> | |
| − | + | <div>[4] Wei, R., Oeser, T., Schmidt, J. et al. (2016). Engineered bacterial polyester hydrolases efficiently degrade polyethylene terephthalate due to relieved product inhibition. Biotechnol Bioeng, 113(8), 1658-1665. doi: 10.1002/bit.25941</div> | |
| − | + | </div> | |
| − | + | <div class="h4-title">Goal</div> | |
| − | + | <div class="sec-wenzi-content">1. Gain PETase protein, repeat the assay of wild-type PETase enzyme activity</div> | |
| − | + | <div class="sec-wenzi-content">2. Gain PETase protein crystal and high-resolution protein tertiary structure based on analysis.</div> | |
| − | + | <div class="sec-wenzi-content">3. Design mutational PETase, filtrate the one which has higher enzyme activity than the wild-type PETase.</div> | |
| − | + | <div class="h4-title">Design</div> | |
| − | + | <div class="tuwen-img-box"> | |
| − | + | <img src="https://static.igem.org/mediawiki/igem.org/d/d6/Description24.jpeg"/> | |
| − | + | <div>PETase&TfCut2 homologous sequence alignment map</div> | |
| − | + | </div> | |
| − | + | <div class="tuwen-img-box"> | |
| − | + | <img src="https://static.igem.org/mediawiki/igem.org/2/21/Description1.jpg"/> | |
| − | + | <div>PETase&Est119 homologous sequence alignment map</div> | |
| − | + | </div> | |
| − | + | <div class="tuwen-img-box"> | |
| − | + | <img src="https://static.igem.org/mediawiki/igem.org/3/31/Description2.jpg"/> | |
| − | + | <div></div> | |
| − | + | </div> | |
| − | + | <div class="clear"></div> | |
| − | + | </div> | |
| − | + | <div class="sec-list-wenzi"> | |
| − | + | <h3 id="part5">Surface Display</h3> | |
| − | + | <div class="h4-title">Background</div> | |
| − | + | <div class="b-No">No.1 Virtue of the Surface Display</div> | |
| − | + | <div class="sec-wenzi-content">Surface display technology is a method of obtaining a whole cell catalyst by fusion of the gene encoding the target protein or polypeptide with the gene of the anchor protein, and post-translating and folding the fusion protein to display the target protein on the surface of the host cell wall. PETase surface display is equivalent to doing the enzyme immobilization, its fixed positioning in the cell surface. Compared with the free enzyme, the immobilized enzyme has the advantages of high stability, easy recovery, easy control, repeated use and low cost. However, the protein purification process is cumbersome, long-term storage of easily degradable and easy to dimerize the impact of enzyme activity, and direct use of whole cell catalytic reaction, will avoid the problem of protein purification.</div> | |
| − | + | <div class="b-No">No2. Option of Fusion Protein Expression System</div> | |
| − | + | <div class="sec-wenzi-content">Vitro expression system of recombinant protein is divided into prokaryotic expression system and eukaryotic expression system. Because the two kinds of expression systems have their own advantages, we decided to do surface display in prokaryotic expression system and eukaryotic expression system respectively in order to compare the expression of PETase in two expression systems is good or bad. In prokaryotic expression system we have selected the most widely used Escherichia coli, which has the characteristics of low cost, short culture period and thorough study of genetic background. In eukaryotic expression system, we select Pichia pastoris. Pichia pastoris could grow and express protein efficiently using methanol as the sole carbon source with the absence of the inhibitor. The promoter of the alcohol oxidase (AOX1) gene in the methanol metabolism pathway of Pichia pastoris is one of the most powerful promoters of eukaryotic promoters. Using the recombinant protein expressed by the AOX1 promoter, the yield can be as high as 20-30 g / L. Moreover, it is more stable than Escherichia coli and has a low mutation rate. The introduced PETase gene will be integrated into the yeast genome, which is more conducive to the stable expression of PETase and the maintenance of enzyme activity.</div> | |
| − | + | <div class="b-No">No3. Selection of Anchor Sequences</div> | |
| − | + | <div class="sec-wenzi-content">We considered a variety of factors, a comprehensive analysis of the characteristics of many kinds of anchored sequences, and ultimately determine the following:</div> | |
| − | + | <div class="h4-title">Escherichia coli</div> | |
| − | + | <div class="tuwen-img-box"> | |
| − | + | <img src="https://static.igem.org/mediawiki/igem.org/1/1d/Description3.jpg"/> | |
| − | + | <div></div> | |
| − | + | </div> | |
| − | + | <div class="h4-title">pichia pastoris</div> | |
| − | + | <div class="tuwen-img-box"> | |
| − | + | <img src="https://static.igem.org/mediawiki/igem.org/4/4e/Description4.jpg"/> | |
| − | + | <div></div> | |
| − | + | </div> | |
| − | + | <div class="references"> | |
| − | + | <div>References:</div> | |
| − | + | <div>[1] Rutherford, Nancy,Mourez, Michael: Surface display of proteins by Gram-negative bacterial autotransporters. MICROBIAL CELL FACTORIES ,2006 5(22)</div> | |
| − | + | <div>[2] Jarmander, Johan; Gustavsson, Martin; Thi-Huyen Do: A dual tag system for facilitated detection of surface expressed proteins in Escherichia coli. MICROBIAL CELL FACTORIES,2012 11(118)</div> | |
| − | + | <div>[3] Karami, Ali; Latifi, Ali Mohamad; Khodi, Samaneh: Comparison of the Organophosphorus Hydrolase Surface Display Using InaVN and Lpp-OmpA Systems in Escherichia coli. JOURNAL OF MICROBIOLOGY AND BIOTECHNOLOGY,2014 24(3) 379-385 </div> | |
| − | + | <div>[4] Li, L; Kang, DG; Cha, HJ: Functional display of foreign protein on surface of Escherichia coli using N-terminal domain of ice nucleation protein. BIOTECHNOLOGY AND BIOENGINEERING,2004 85(2) 214-221 </div> | |
| − | + | <div>[5] Zhai Y, Zhang K, Huo Y, Zhu Y, Zhou Q, Lu J, Black I, Pang X, Roszak AW, Zhang X et al (2011) Autotransporter passenger domain secretion requires a hydrophobic cavity at the extracellular entrance of the betadomain pore. Biochem J 435:577–587 </div> | |
| − | + | <div>[6] Dautin N, Barnard TJ, Anderson DE, Bernstein HD (2007) Cleavage of a bacterial autotransporter by an evolutionarily convergent autocatalytic mechanism. EMBO J 26:1942–1952 </div> | |
| − | + | <div>[7] Fang S, Pang X, Tian X, et al. BrkAutoDisplay: functional display of multiple exogenous proteins on the surface of Escherichia coli, by using BrkA autotransporter[J]. Microbial Cell Factories, 2014, 14(1):1-12.</div> | |
| − | + | <div>[8] DongHeng Guo, YanShan Xu, YaJun Kang et al (2016). Synthesis of octyl-β- d -glucopyranoside catalyzed by Thai rosewood β-glucosidase - displaying Pichia pastoris, in an aqueous/organic two-phase system[J]. Enzyme & Microbial Technology, 2016, 85:90–97.</div> | |
| − | + | </div> | |
| − | + | <div class="h4-title">Goal</div> | |
| − | + | <div class="sec-wenzi-content">1. Achieving separately PETase surface display successful on E. coli BL21 cell surface with using of four different anchor sequences, AIDA, BrkA, LPP-OmpA, INP, access to whole cell catalyst.</div> | |
| − | + | <div class="sec-wenzi-content">2. Achieving separately PETase surface display successful on Pichia pastoris GS115 cell surface with using of three different anchor sequences, GCW21, GCW51, GCW61, access to whole cell catalyst.</div> | |
| − | + | <div class="sec-wenzi-content">3. Screening out the optimal conditions for the carrier expression constructed by four different anchor sequences, AIDA, BrkA, LPP-OmpA, INP, in E.coli BL21 and for PET degradation.</div> | |
| − | + | <div class="sec-wenzi-content">4. Screening out the optimal conditions for the carrier expression constructed by three different anchor sequences, GCW21, GCW51, GCW61, in Pichia pastoris GS115 and for PET degradation.</div> | |
| − | + | <div class="h4-title">Design</div> | |
| − | + | <div class="tuwen-img-box"> | |
| − | + | <img src="https://static.igem.org/mediawiki/igem.org/3/34/Description35.jpg"/> | |
| − | + | <div>Escherichia coli expression vector x5 (INP x2)</div> | |
| − | + | </div> | |
| − | + | <div class="tuwen-img-box"> | |
| − | + | <img src="https://static.igem.org/mediawiki/igem.org/e/ec/Description28.jpg"/> | |
| − | + | <div>Surface display of E.coli schematic diagram</div> | |
| − | + | </div> | |
| − | + | <div class="tuwen-img-box"> | |
| − | + | <img src="https://static.igem.org/mediawiki/igem.org/6/6d/Description36.jpg"/> | |
| − | + | <div>Pichia pastoris expression vector x3</div> | |
| − | + | </div> | |
| − | + | <div class="tuwen-img-box"> | |
| − | + | <img src="https://static.igem.org/mediawiki/igem.org/0/0e/Description30.jpg"/> | |
| − | + | <div>Surface display of Pichia pastoris schematic diagram</div> | |
| − | + | </div> | |
| − | + | <div class="clear"></div> | |
| − | + | </div> | |
| − | + | <div class="sec-list-wenzi"> | |
| − | + | <h3 id="part6">Fusing secretion of hydrophobin and PETase and their co - display</h3> | |
| − | + | <div class="sec-wenzi-content">Hydrophobin is a small molecular weight protein produced by filamentous fungi, which is characterized by the ability to form a layer of amphiphilic protein membrane at the interface in order to reverse the nature of the interface covered. Hydrophobin amphipathicity refers to the hydrophobic nature that the hydrophilic end of a hydrophobin. The hydrophobic protein and PETase fuse fusion protein, whose characteristics are adsorption of PET film and PET film properties degradation.</div> | |
| − | + | <div class="sec-wenzi-content">The yeast cell wall is hydrophilic while the hydrophobic protein is hydrophobic. Due to its amphiphilic nature, the hydrophobic protein binds to the surface of yeast cells spontaneously and binds the hydrophobic surface toward the extracellular side, which adsorbs the PET film through the hydrophobic interaction force. Utilized amphiphilic and high temperature, acid and alkali resistance characteristics of hydrophobic protein, co-display the PETase and hydrophobic protein on the surface of eukaryotic cells helps in greatly improving the enzyme activity and broaden the reaction conditions of the enzyme.</div> | |
| − | + | <div class="sec-wenzi-content">At present, result in yeast industrial fermentation technology matures, using yeast as the host cell is conducive to the future industrial applications.</div> | |
| − | + | <div class="references"> | |
| − | + | <div>References:</div> | |
| − | + | <div>[1] Pan W, Jie H, Sun Y, et al. Display of fungal hydrophobin on the Pichia pastoris cell surface and its influence on Candida antarctica lipase B[J]. Applied Microbiology & Biotechnology, 2016:1-13.</div> | |
| − | + | </div> | |
| − | + | <div class="h4-title">Goal</div> | |
| − | + | <div class="sec-wenzi-content">1. Using Pichia pastoris GS115 secrete PETase alone. Compare the PETase activity from eukaryotic and prokaryotic cells in order to know if the expression of prokaryotic protein can be positive in eukaryotic cells.</div> | |
| − | + | <div class="sec-wenzi-content">2. Using Pichia pastoris GS115 secrete fusion protein combined by PETase and two kinds of hydrophobic protein, sJanus and inJanus. Compared with the PETasse secretion in eukaryotic alone, whether the effects of the fusion protein of PETase and hydrophobin on the enzyme activity were determined</div> | |
| − | + | <div class="sec-wenzi-content">3 Two hydrophobic proteins, sJanus and inJanus, were successfully screened on the surface of Pichia pastoris GS115 using the GCW61 anchor sequence as the control group of co-display.</div> | |
| − | + | <div class="sec-wenzi-content">4 Screening out the optimal expression conditions of the yeast transformants co-displayed by PETase and two different kinds of hydrophobin, sJanus and inJanus, and the optimum reaction conditions for the PET degradation</div> | |
| − | + | <div class="h4-title">Design</div> | |
| − | + | <div class="tuwen-img-box"> | |
| − | + | <img src="https://static.igem.org/mediawiki/igem.org/9/95/Description13.jpg"/> | |
| − | + | <div>Separate secretion of PETase</div> | |
| − | + | </div> | |
| − | + | <div class="tuwen-img-box"> | |
| − | + | <img src="https://static.igem.org/mediawiki/igem.org/9/9f/Description37.jpg"/> | |
| − | + | <div>Fusion of hydrophobins (sJanus/ inJanus) and PETase</div> | |
| − | + | </div> | |
| − | + | <div class="tuwen-img-box"> | |
| − | + | <img src="https://static.igem.org/mediawiki/igem.org/b/be/Description38.jpg"/> | |
| − | + | <div>Separate display of hydrophobins (sJanus/ inJanus)</div> | |
| − | + | </div> | |
| − | + | <div class="tuwen-img-box"> | |
| − | + | <img src="https://static.igem.org/mediawiki/igem.org/1/11/Description39.jpg"/> | |
| − | + | <div>Co-display of PETase and hydrophobins (sJanus/ inJanus (2x3=6 kinds)</div> | |
| − | + | </div> | |
| − | + | <div class="tuwen-img-box"> | |
| − | + | <img src="https://static.igem.org/mediawiki/igem.org/0/07/Description31.jpg"/> | |
| − | + | <div>Fusing secretion of hydrophobin and PETase and their co - display schematic diagram</div> | |
| − | + | </div> | |
| − | + | </div> | |
| − | + | <div class="up"><img src="https://static.igem.org/mediawiki/2016/6/62/T--TJUSLS_China--up.jpg"/></div> | |
| − | + | </div> | |
| − | + | <!--foot--> | |
| − | + | <div class="footer_bg"> | |
| − | + | <div class="foot_wrap"> | |
| − | + | <div class="footer"> | |
| − | + | ||
| − | + | <div class="footer_btm"> | |
| − | + | ||
| − | + | <div class="copy"> | |
| − | + | <p class="w3-link">Copyright 2013 <span><a href="index.html"> TJUSLS</a></span>. Template by <a href="http://w3layouts.com/"> W3Layouts</a></p> | |
| − | + | </div> | |
| − | + | <div class="clear"></div> | |
| − | + | </div> | |
| − | + | </div> | |
| − | + | </div> | |
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
| − | + | ||
</div> | </div> | ||
</body> | </body> | ||
| − | |||
</html> | </html> | ||
Revision as of 07:21, 18 October 2016
Description


Overview
We put forward a new model about enzymology called Training Protein. It can be applied on every enzyme. According to its self-characteristic, people will do targeted modify on the enzyme in order to an extensive application.
Frist and the foremost, researchers should analyze the protein tertiary structure and understand its characteristic for well-directed proceeding to the next section. The next step has two paths, one is directed mutation, which site is chosen by analyzing the protein tertiary structure to find its functional domains, and the other is surface display. Surface display can improve the catalytic efficiency of the enzyme, and avoid the trouble of protein purification.
Application
TJUSLS China's subject of the competition for this year is to modify PET hydrolase (PETase) and developing cell surface display. PETase was found from a kind of microorganism living on PET as the main carbon source. It can degrade macromolecular polymers into monomers. Surface display can reveal the protein whose gene code is coalescing the gene code of target protein or polypeptide with the counterpart of ankyrin on the surface of the host cell wall to harvest the whole cell catalyst. The protagonists of our project, which are PETase and the surface display technology, will act in three following aspects.
Firstly, we find the enzyme catalytic center and its binding center by analyzing the structure of PETase with Protein crystallography and X ray diffraction technique, as well as choosing the mutation site under its character direction in order to carry out the directional mutation to improve the degradation efficiency and thermal stability. Moreover, using the prokaryotic (E. coli) and eukaryotic (Pichia Pastoris) surface display for whole cell catalysis. Thirdly, fusing the PETase and hydrophobic protein then expressing the fusion protein in Pichia Pastoris, which will take advantage of hydrophobic protein in hydrophobicity to give a hydrophobic environment for a better degradation efficiency. Meanwhile, the co-display combining PETase and hydrophobic protein in Pichia Pastoris will change the character of cells' surfaces so that cells can adapt the extreme environment, then the whole-cell biocatalyst might have higher catalytic efficiency as well as break the limitation set by reaction condition. According to that, the PETase degradation reaction conditions will be broaden which means it can be applied in industry easier.
Background
Polyethyleneterephthalate (PET) is the condensation of terephthalic acid and ethylene glycol.
PET is mainly used as fiber in textile industry, sometime applying in film and engineering plastic. PET film can be used as electrical insulating material like capacitor, cable insulation, printed wiring substrate, electrode slot insulation, etc. The second application is base or baseband, as film, X-ray, tape, computer tape, etc. PET film can change into metallic films by vacuum aluminizing, like gold and silver wire, micro capacitor film, etc. The third direction is blow-molding products, for example tensile polyester bottle using in packing.
With the increasing demand of the society, because of its good fastness weather, electrical insulation, and having the friction resistance, high strength, good transparency, non-toxic, anti penetration, light quality, high production efficiency and some others advantages, the rapid growth of PET production and sales is consequent, which leads to the result that more and more obsolete PET has already caused the environment problem.
According to the report from Research and Markets, the PET global market will shows annual growth of 7.3 percent during 2014 to 2019. Its market value is expected to reach about 47 billion 400 million dollars until 2019. However, the obsolete PET, which will take up a lot of space and cause white pollution, has strong chemical inertia and difficult in degradation. In a word, PET degradation is the most urgent problem.
The mainly methods are landfill, burning and recycle. Landfill and burning are too simple to protect the environment. So degradation and recycling will be a better choice, like physical recycling, chemical degradation and biodegradation.
The physical recycling is a plastic reprocessing and recycling technology by cutting, smashing, Heating and melting. Although the physical way is low cost, it will cause the regeneration product performance delays with impurities accumulate. So it can only be degrade in using.
PET is fully dense and high crystallinity with ester bond in molecular chain, which is possible degraded. Under the high temperature, PET reacts with chemical reagents, called depolymerizing reaction, and turns into smaller molecule, intermediate raw material or units. Products can reuse as the crude material of PET after separation and purification. Even if chemical degradation realize the recycling of raw materials, it might cause equipment corrosion seriously as well as increasing energy and reagent consumption, which is hardly mean eco-friendly.
In the other words, biodegradation, which means taking advantages of microorganism, such as bacteria and fungus, to degraded plastic, has broad application prospects result from its low cost and high degradation efficiency.
Structure and mutation
Background
PETase is the only enzyme found in bacteria which can degrade PET. Compare to the other enzyme found in fungi like LCC, TfH, FsC, PETase is much more active under low temperature environment (20~40℃), which means its reaction conditions is feasible in practical application than the others'. Besides,PETase is good at degrading highly-crystallized PET (such as plastic bottles) while others don’t, their enzyme activity to highly-crystallized PET is more than 20 times lower than PETase.However, we should not deny that the degradation rate of PETase is not enough for put on stream because of its activity. To get over this barrier, we manage to enhance its activity by site-direxted mutagenesis. The tertiary structure of a protein is closely coupled to its function. The tertiary structure of a protein is experimentally determined, predominantly by two methods called X-ray Diffraction and Protein Crystallography. According to that, we find the catalytic center and combination center of the enzyme, which will assist us to know how the enzyme works, and choose mutation sites on the basis of that.

Compare to the other enzyme found in fungi like LCC, TfH, FsC, PETase is much more active under low temperature environment (20~40℃)
References:
[1] Yoshida S, Hiraga K, Takehana T, et al. A bacterium that degrades and assimilates poly(ethylene terephthalate).[J]. Science, 2016, 351(6278):1196-1199.
[2] Kitadokoro, Kengo, Thumarat et al.(2012). Crystal structure of cutinase Est119 from Thermobifida alba AHK119 that can degrade modified polyethylene terephthalate at 1.76Å resolution. Polymer Degradation and Stability, 97(5), 771-775. doi: 10.1016/j.polymdegradstab.2012.02.003
[3] Roth, C., Wei, R., Oeser, T. et al. (2014). Structural and functional studies on a thermostable polyethylene terephthalate degrading hydrolase from Thermobifida fusca. Appl Microbiol Biotechnol, 98(18), 7815-7823. doi: 10.1007/s00253-014-5672-0
[4] Wei, R., Oeser, T., Schmidt, J. et al. (2016). Engineered bacterial polyester hydrolases efficiently degrade polyethylene terephthalate due to relieved product inhibition. Biotechnol Bioeng, 113(8), 1658-1665. doi: 10.1002/bit.25941
Goal
1. Gain PETase protein, repeat the assay of wild-type PETase enzyme activity
2. Gain PETase protein crystal and high-resolution protein tertiary structure based on analysis.
3. Design mutational PETase, filtrate the one which has higher enzyme activity than the wild-type PETase.
Design

PETase&TfCut2 homologous sequence alignment map
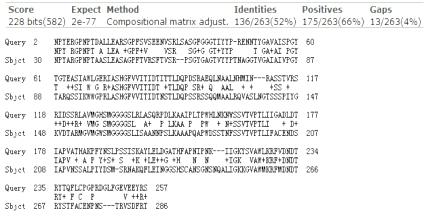
PETase&Est119 homologous sequence alignment map

Surface Display
Background
No.1 Virtue of the Surface Display
Surface display technology is a method of obtaining a whole cell catalyst by fusion of the gene encoding the target protein or polypeptide with the gene of the anchor protein, and post-translating and folding the fusion protein to display the target protein on the surface of the host cell wall. PETase surface display is equivalent to doing the enzyme immobilization, its fixed positioning in the cell surface. Compared with the free enzyme, the immobilized enzyme has the advantages of high stability, easy recovery, easy control, repeated use and low cost. However, the protein purification process is cumbersome, long-term storage of easily degradable and easy to dimerize the impact of enzyme activity, and direct use of whole cell catalytic reaction, will avoid the problem of protein purification.
No2. Option of Fusion Protein Expression System
Vitro expression system of recombinant protein is divided into prokaryotic expression system and eukaryotic expression system. Because the two kinds of expression systems have their own advantages, we decided to do surface display in prokaryotic expression system and eukaryotic expression system respectively in order to compare the expression of PETase in two expression systems is good or bad. In prokaryotic expression system we have selected the most widely used Escherichia coli, which has the characteristics of low cost, short culture period and thorough study of genetic background. In eukaryotic expression system, we select Pichia pastoris. Pichia pastoris could grow and express protein efficiently using methanol as the sole carbon source with the absence of the inhibitor. The promoter of the alcohol oxidase (AOX1) gene in the methanol metabolism pathway of Pichia pastoris is one of the most powerful promoters of eukaryotic promoters. Using the recombinant protein expressed by the AOX1 promoter, the yield can be as high as 20-30 g / L. Moreover, it is more stable than Escherichia coli and has a low mutation rate. The introduced PETase gene will be integrated into the yeast genome, which is more conducive to the stable expression of PETase and the maintenance of enzyme activity.
No3. Selection of Anchor Sequences
We considered a variety of factors, a comprehensive analysis of the characteristics of many kinds of anchored sequences, and ultimately determine the following:
Escherichia coli

pichia pastoris

References:
[1] Rutherford, Nancy,Mourez, Michael: Surface display of proteins by Gram-negative bacterial autotransporters. MICROBIAL CELL FACTORIES ,2006 5(22)
[2] Jarmander, Johan; Gustavsson, Martin; Thi-Huyen Do: A dual tag system for facilitated detection of surface expressed proteins in Escherichia coli. MICROBIAL CELL FACTORIES,2012 11(118)
[3] Karami, Ali; Latifi, Ali Mohamad; Khodi, Samaneh: Comparison of the Organophosphorus Hydrolase Surface Display Using InaVN and Lpp-OmpA Systems in Escherichia coli. JOURNAL OF MICROBIOLOGY AND BIOTECHNOLOGY,2014 24(3) 379-385
[4] Li, L; Kang, DG; Cha, HJ: Functional display of foreign protein on surface of Escherichia coli using N-terminal domain of ice nucleation protein. BIOTECHNOLOGY AND BIOENGINEERING,2004 85(2) 214-221
[5] Zhai Y, Zhang K, Huo Y, Zhu Y, Zhou Q, Lu J, Black I, Pang X, Roszak AW, Zhang X et al (2011) Autotransporter passenger domain secretion requires a hydrophobic cavity at the extracellular entrance of the betadomain pore. Biochem J 435:577–587
[6] Dautin N, Barnard TJ, Anderson DE, Bernstein HD (2007) Cleavage of a bacterial autotransporter by an evolutionarily convergent autocatalytic mechanism. EMBO J 26:1942–1952
[7] Fang S, Pang X, Tian X, et al. BrkAutoDisplay: functional display of multiple exogenous proteins on the surface of Escherichia coli, by using BrkA autotransporter[J]. Microbial Cell Factories, 2014, 14(1):1-12.
[8] DongHeng Guo, YanShan Xu, YaJun Kang et al (2016). Synthesis of octyl-β- d -glucopyranoside catalyzed by Thai rosewood β-glucosidase - displaying Pichia pastoris, in an aqueous/organic two-phase system[J]. Enzyme & Microbial Technology, 2016, 85:90–97.
Goal
1. Achieving separately PETase surface display successful on E. coli BL21 cell surface with using of four different anchor sequences, AIDA, BrkA, LPP-OmpA, INP, access to whole cell catalyst.
2. Achieving separately PETase surface display successful on Pichia pastoris GS115 cell surface with using of three different anchor sequences, GCW21, GCW51, GCW61, access to whole cell catalyst.
3. Screening out the optimal conditions for the carrier expression constructed by four different anchor sequences, AIDA, BrkA, LPP-OmpA, INP, in E.coli BL21 and for PET degradation.
4. Screening out the optimal conditions for the carrier expression constructed by three different anchor sequences, GCW21, GCW51, GCW61, in Pichia pastoris GS115 and for PET degradation.
Design

Escherichia coli expression vector x5 (INP x2)

Surface display of E.coli schematic diagram

Pichia pastoris expression vector x3

Surface display of Pichia pastoris schematic diagram
Fusing secretion of hydrophobin and PETase and their co - display
Hydrophobin is a small molecular weight protein produced by filamentous fungi, which is characterized by the ability to form a layer of amphiphilic protein membrane at the interface in order to reverse the nature of the interface covered. Hydrophobin amphipathicity refers to the hydrophobic nature that the hydrophilic end of a hydrophobin. The hydrophobic protein and PETase fuse fusion protein, whose characteristics are adsorption of PET film and PET film properties degradation.
The yeast cell wall is hydrophilic while the hydrophobic protein is hydrophobic. Due to its amphiphilic nature, the hydrophobic protein binds to the surface of yeast cells spontaneously and binds the hydrophobic surface toward the extracellular side, which adsorbs the PET film through the hydrophobic interaction force. Utilized amphiphilic and high temperature, acid and alkali resistance characteristics of hydrophobic protein, co-display the PETase and hydrophobic protein on the surface of eukaryotic cells helps in greatly improving the enzyme activity and broaden the reaction conditions of the enzyme.
At present, result in yeast industrial fermentation technology matures, using yeast as the host cell is conducive to the future industrial applications.
References:
[1] Pan W, Jie H, Sun Y, et al. Display of fungal hydrophobin on the Pichia pastoris cell surface and its influence on Candida antarctica lipase B[J]. Applied Microbiology & Biotechnology, 2016:1-13.
Goal
1. Using Pichia pastoris GS115 secrete PETase alone. Compare the PETase activity from eukaryotic and prokaryotic cells in order to know if the expression of prokaryotic protein can be positive in eukaryotic cells.
2. Using Pichia pastoris GS115 secrete fusion protein combined by PETase and two kinds of hydrophobic protein, sJanus and inJanus. Compared with the PETasse secretion in eukaryotic alone, whether the effects of the fusion protein of PETase and hydrophobin on the enzyme activity were determined
3 Two hydrophobic proteins, sJanus and inJanus, were successfully screened on the surface of Pichia pastoris GS115 using the GCW61 anchor sequence as the control group of co-display.
4 Screening out the optimal expression conditions of the yeast transformants co-displayed by PETase and two different kinds of hydrophobin, sJanus and inJanus, and the optimum reaction conditions for the PET degradation
Design

Separate secretion of PETase

Fusion of hydrophobins (sJanus/ inJanus) and PETase

Separate display of hydrophobins (sJanus/ inJanus)

Co-display of PETase and hydrophobins (sJanus/ inJanus (2x3=6 kinds)

Fusing secretion of hydrophobin and PETase and their co - display schematic diagram




