Crepuscule (Talk | contribs) |
Crepuscule (Talk | contribs) |
||
| Line 298: | Line 298: | ||
Two of the successful ones were used to inoculate overnight cultures.<br/> | Two of the successful ones were used to inoculate overnight cultures.<br/> | ||
| − | <img align="center" src="https://static.igem.org/mediawiki/2016/8/81/Igem-6803-week4.png" alt="Igem-6803-week4 | + | <img align="center" src="https://static.igem.org/mediawiki/2016/8/81/Igem-6803-week4.png" alt="Igem-6803-week4" > <br/> |
</a> | </a> | ||
Revision as of 06:39, 14 October 2016
Week2(8/14/2016-8/20/2016)
Synechococcus sp PCC 7942 had no signals of life.

Week3(8/28/2016-9/3/2016)
Aug.29th
Aug.30th
PCR worked, positive control worked, no amplification of 19.
15 amplification at 65.0°C with 15.rev/fwd primes.
PCR worked, positive control worked, no amplification of 15.
Aug.31th
Sep.1th
3.Two of these colonies containing 19 were used to inoculate overnight cultures.
4.15 gene fragment was phosphorylated.
Sep.2th
1.Plasmids containing T vector with 19(pT-19)were isolated using a miniprep kit.
2.Mono-restriction digest of pT-19 with stu I.
3.The enzyme-digested product was dephosphorylation.
4.Dephosphorylated plasmid and phosphorylated gene 15 were connected.
5.Ligation product was transformed into E.coli via heat shock.
2.Mono-restriction digest of pT-19 with stu I.
3.The enzyme-digested product was dephosphorylation.

4.Dephosphorylated plasmid and phosphorylated gene 15 were connected.
5.Ligation product was transformed into E.coli via heat shock.
Sep.3th
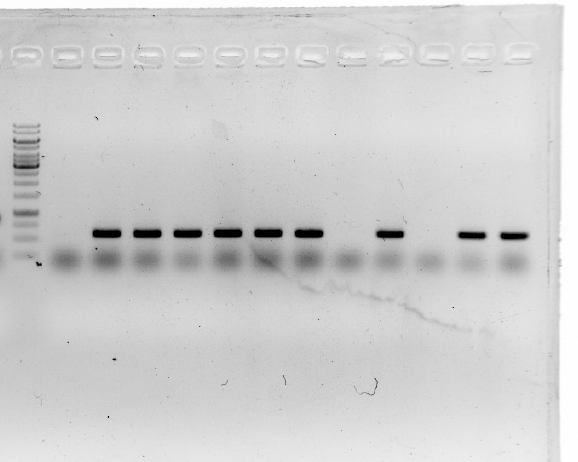
1.A colony PCR was performed with twelve colonies.
2.Gel electrophoresis showed that 2 colonies were positive for connecting the plasmid and gene fragment.
3.These two colonies were used to inoculate overnight cultures.
2.Gel electrophoresis showed that 2 colonies were positive for connecting the plasmid and gene fragment.
3.These two colonies were used to inoculate overnight cultures.
Week4(9/4/2016-9/10/2016)
Sep.4th
1.Plasmids with correct sequence of 19-15 were isolated using a miniprep kit.
2.Mono-restriction digest of pT-19-15 with Nru I.
3.The enzyme-digested product was dephosphorylation.
2.Mono-restriction digest of pT-19-15 with Nru I.
3.The enzyme-digested product was dephosphorylation.
Sep.6th
Colonies containing gene 13 were used to inoculate overnight cultures.
Sep.7th
1.Plasmids were isolated using a miniprep kit.
2.Amplification of 13 with Q5 High-Fidelity DNA Polymerase out of E.coli
3.13 amplification at 65.0°C with 13.rev/fwd primes
PCR worked, positive control worked, no amplification of 13
4.The fragments of 13 were purified with PCR Purification Kit.
2.Amplification of 13 with Q5 High-Fidelity DNA Polymerase out of E.coli
3.13 amplification at 65.0°C with 13.rev/fwd primes
PCR worked, positive control worked, no amplification of 13
4.The fragments of 13 were purified with PCR Purification Kit.
Sep.8th
13 gene fragment was phosphorylated.
Sep.9th
1.Insertion of Ni promoter and ligation of 13-19-15
2.Ni inducible promoter was ligated into pCPC-3301 vector.
3.Phosphorylated 13 was ligated into pT-19-15 and transformed into E.coli via heat shock.
4.Single colonies were obtained by plating.
2.Ni inducible promoter was ligated into pCPC-3301 vector.
3.Phosphorylated 13 was ligated into pT-19-15 and transformed into E.coli via heat shock.
4.Single colonies were obtained by plating.
Sep.10th
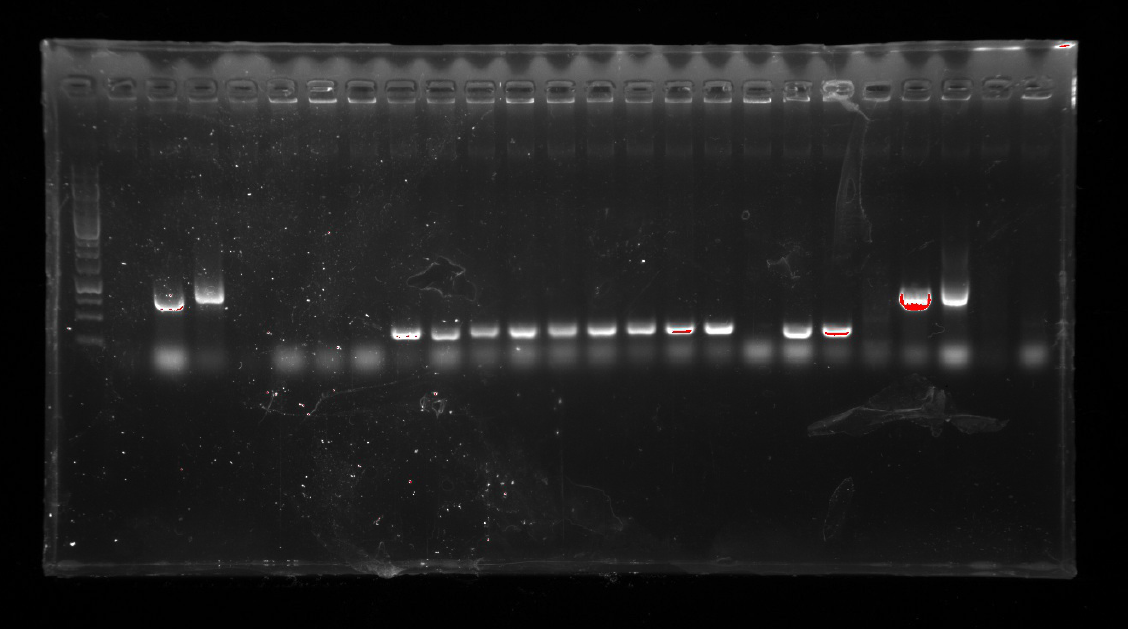
1.A colony PCR of Ni inducible promoter(pCPC-3301-Ni) was performed with 12 colonies.
Two of the successful ones were used to inoculate overnight cultures.
2.A colony PCR of pT-13-19-15 was performed with 7 colonies.
Two of the successful ones were used to inoculate overnight cultures.
Two of the successful ones were used to inoculate overnight cultures.
2.A colony PCR of pT-13-19-15 was performed with 7 colonies.
Two of the successful ones were used to inoculate overnight cultures.

Week5(9/11/2016-9/17/2016)
Sep.11th
Two kinds of plasmids were isolated using a miniprep kit.
Sep.12th
Two kinds of plasmids were isolated using a miniprep kit.
Sep.14th
The sequencing results for both of them were error.
Sep.15th
1.Colonies containing Ni inducible promoter were used to inoculate overnight cultures.
2.PCR was performed to check if the gene fragments were ligated correctly.
13_ fwd and 15_rev on pT-13-19-15
3.Gel electrophoresis showed that it failed.
2.PCR was performed to check if the gene fragments were ligated correctly.
13_ fwd and 15_rev on pT-13-19-15
3.Gel electrophoresis showed that it failed.
Sep.16th
Plasmids pCPC-3031-Ni were isolated using a miniprep kit.
Sep.17th
1.Several PCRs were performed to check if the gene fragments were ligated correctly.
13_fwd and 13_rev on pT-13-19-15
19_fwd and 19_rev on pT-13-19-15
15_fwd and 15_rev on pT-13-19-15
13_fwd and 19_rev on pT-13-19-15
19_fwd and 15_rev on pT-13-19-15
13_ wd and 15_rev on pT-13-19-15
The fourth and sixth ones were not successful.

2.Repetition: Phosphorylated 13 was ligated into pT-19-15 and transformed into E.coli via heat shock.
13_fwd and 13_rev on pT-13-19-15
19_fwd and 19_rev on pT-13-19-15
15_fwd and 15_rev on pT-13-19-15
13_fwd and 19_rev on pT-13-19-15
19_fwd and 15_rev on pT-13-19-15
13_ wd and 15_rev on pT-13-19-15
The fourth and sixth ones were not successful.

2.Repetition: Phosphorylated 13 was ligated into pT-19-15 and transformed into E.coli via heat shock.
Week6(9/18/2016-9/24/2016)
Sep.18th
Repetition: Several PCRs were performed to check if the gene fragments were ligated correctly.
13_fwd and 13_rev on pT-13-19-15
13_fwd and 19_rev on pT-13-19-15
The second one was failed.
13_fwd and 13_rev on pT-13-19-15
13_fwd and 19_rev on pT-13-19-15
The second one was failed.
Sep.21th
1.Medium preparation :BG-11.
2.19_ fwd and 15_rev were used to amplify 19-15.
2.19_ fwd and 15_rev were used to amplify 19-15.
Sep.22th
1.Gel electrophoresis showed that amplification of fragments was successfull.
2.Ligated 13 and 19-15 via overlap PCR.
3.This PCR worked well and the products were extracted from the gel using a Agarose Gel DNA Extration Kit.
4.Restriction digest on pCPC-3031-Ni with Sac I.

5.The fragment 13-19-15 was ligated onto T vector and transformed into E.coli via heat shock.

2.Ligated 13 and 19-15 via overlap PCR.
3.This PCR worked well and the products were extracted from the gel using a Agarose Gel DNA Extration Kit.
4.Restriction digest on pCPC-3031-Ni with Sac I.

5.The fragment 13-19-15 was ligated onto T vector and transformed into E.coli via heat shock.

Sep.23th
1.A colony PCR of pT-13-19-15 was performed with 12 colonies.
2.Two of these colonies containing pT-13-19-15 were used to inoculate overnight cultures.
3.13-19-15 gene fragment was phosphorylated.
4.Phosphorylated 13-19-15 was ligated into pCPC-3031-Ni and transformed into E.coli via heat shock.
2.Two of these colonies containing pT-13-19-15 were used to inoculate overnight cultures.
3.13-19-15 gene fragment was phosphorylated.
4.Phosphorylated 13-19-15 was ligated into pCPC-3031-Ni and transformed into E.coli via heat shock.
Sep.24th
Plasmids pT-13-19-15 were isolated using a miniprep kit.
Week7(9/25/2016-10/1/2016)
Sep.25th
1.A colony PCR of pCPC-3031-Ni-13-19-15 was performed with 12 colonies.
2.Three colonies were proved to be true. And two of them were used to inoculate overnight cultures.

2.Three colonies were proved to be true. And two of them were used to inoculate overnight cultures.

Sep.26th
Plasmids pT-13-19-15 were handed in for sequencing, which confirmed its correctness.
The sequencing results for them were correct.
The sequencing results for them were correct.
Notice: This page is currently under construction. Contents in this page are temporaory and will be modified several times before the final release. — 2016 iGEM Team Tianjin


