Notes For Modified Cyanobacteria:A Controllable Lipid Producer
Week1(7/31/2016-8/6/2016)
Week2(8/14/2016-8/20/2016)
Week3(8/28/2016-9/3/2016)
Aug.29th
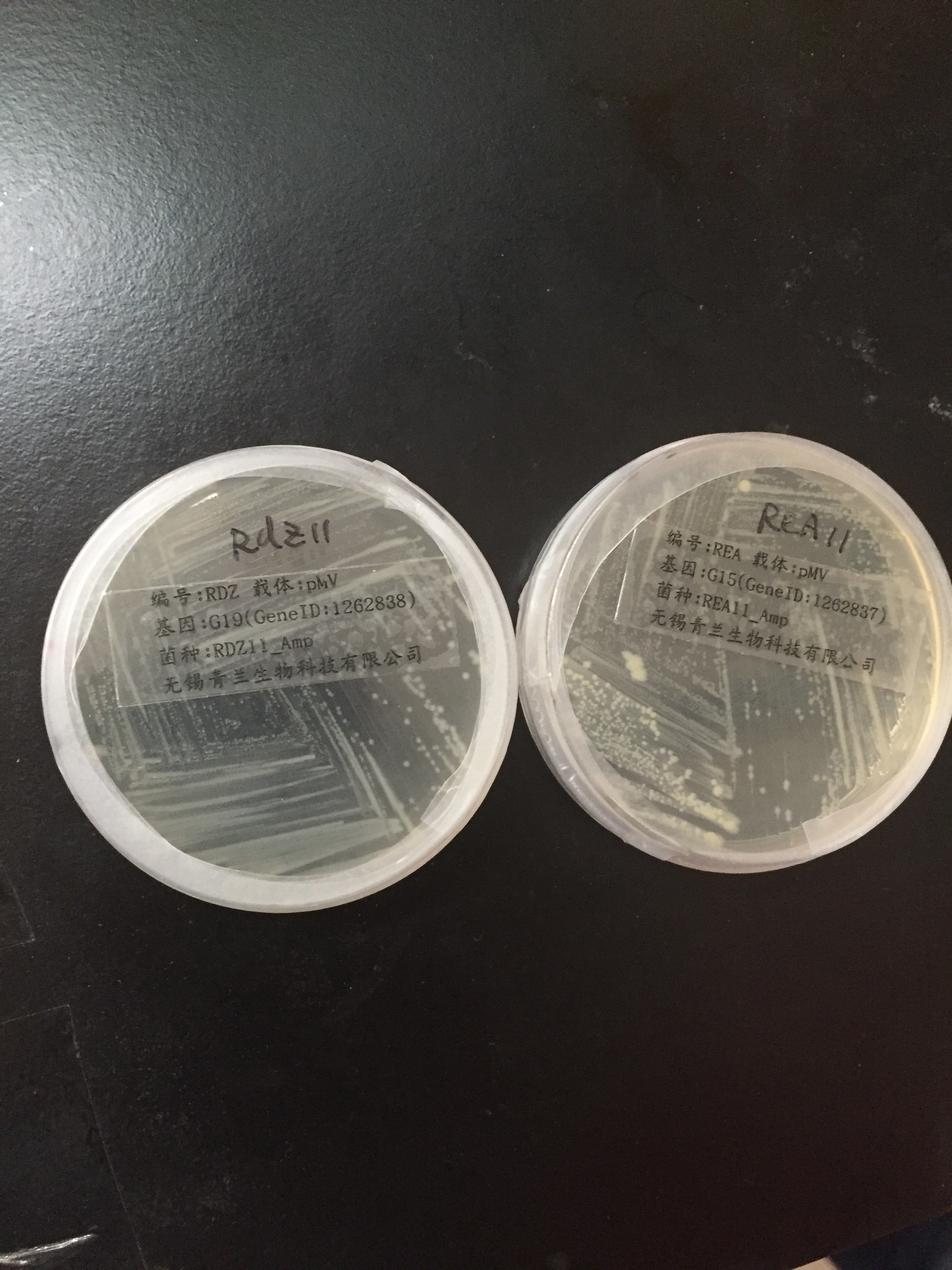
Aug.30th
PCR worked, positive control worked, no amplification of 19.
15 amplification at 65.0°C with 15.rev/fwd primes.
PCR worked, positive control worked, no amplification of 15.
Aug.31th
Sep.1th
Sep.2th
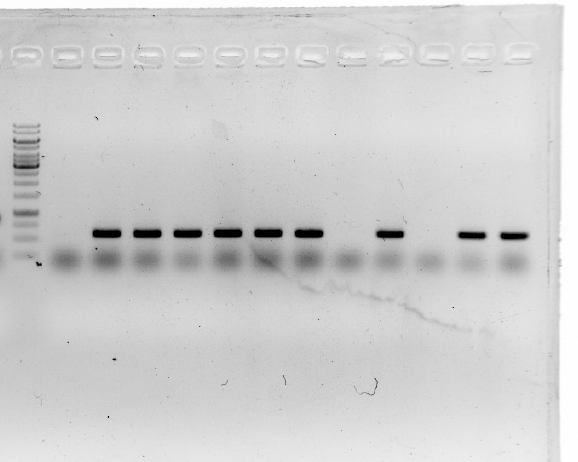
Sep.3th
Week4(9/4/2016-9/10/2016)
Sep.4th
Sep.6th
Sep.7th
Sep.8th
Sep.9th
Sep.10th
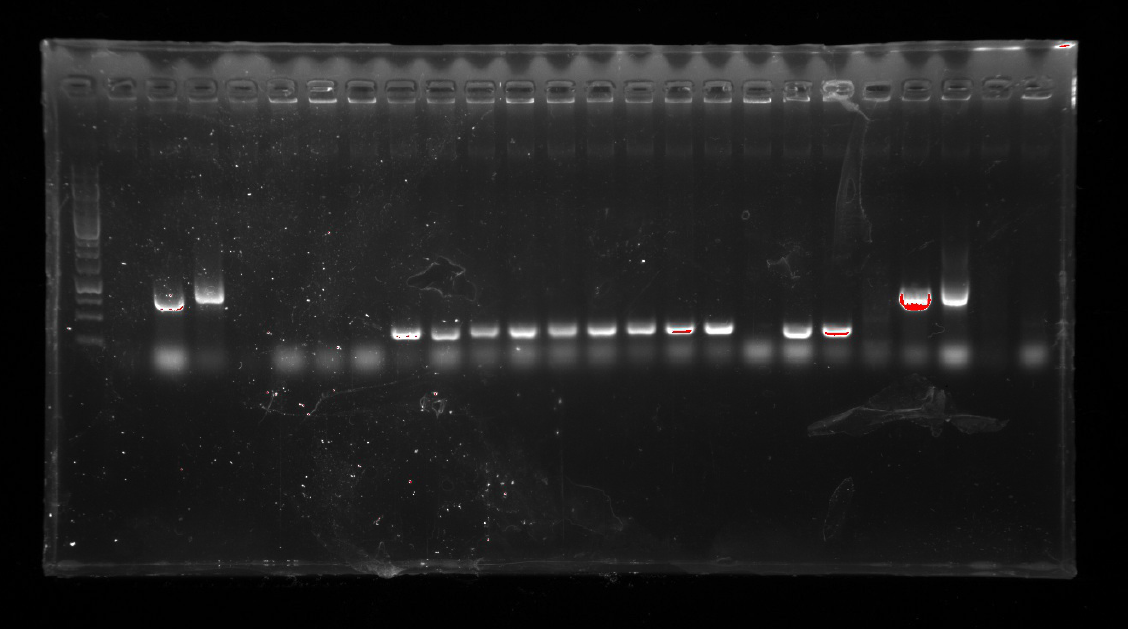
Week5(9/11/2016-9/17/2016)
Sep.11th
Sep.14th
Sep.15th
Sep.16th
Sep.17th
19_fwd and 19_rev on pT-13-19-15
15_fwd and 15_rev on pT-13-19-15
13_fwd and 19_rev on pT-13-19-15
19_fwd and 15_rev on pT-13-19-15
13_ wd and 15_rev on pT-13-19-15
The fourth and sixth ones were not successful.

Week6(9/18/2016-9/24/2016)
Sep.18th
Repetition: Several PCRs were performed to check if the gene fragments were ligated correctly.
13_fwd and 13_rev on pT-13-19-15
13_fwd and 19_rev on pT-13-19-15
The second one was failed.
13_fwd and 13_rev on pT-13-19-15
13_fwd and 19_rev on pT-13-19-15
The second one was failed.
Sep.21th
Sep.22th


Sep.23th
Sep.24th
Week7(9/25/2016-10/1/2016)
Sep.25th





