Crepuscule (Talk | contribs) |
|||
| Line 122: | Line 122: | ||
<br/> | <br/> | ||
Fig.4. Relative activities of enzymes | Fig.4. Relative activities of enzymes | ||
| + | </p> | ||
| + | <div class="col-md-2"></div> | ||
| + | |||
| + | |||
| + | </div> | ||
| + | |||
| + | </div> | ||
| + | |||
| + | |||
| + | <div class="col-md-8"> | ||
| + | <img src="https://static.igem.org/mediawiki/2016/0/02/T--Tianjin--cf-r-5.png" alt="desktop"> | ||
| + | <p style="font-size:15px"> | ||
| + | <br/> | ||
| + | Fig.5. Relative activities of enzymes | ||
</p> | </p> | ||
<div class="col-md-2"></div> | <div class="col-md-2"></div> | ||
Revision as of 14:08, 15 October 2016
Results of CFPS
Overview
We utilized the cell-free system to express the enzymes which had been modified in 22 different sites. Then we used the proteins successfully expressed to degrade PET. Our expected goal was to screen mutations with higher enzyme activities than the wild type PETase. .
Detailed results
After static reaction at 39℃ for 5 days, we detected the characteristic adsorption peak of the product ,MHET, which has no other characteristic adsorption peak except in 260nm.

Fig.1. Spectral scan for the degradation product MHET

Fig.2.Spectral scan for the degradation product MHET(Samples with no other absorption peak except in 260nm)
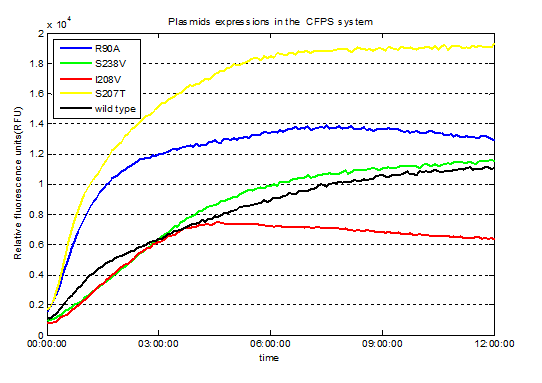
Fig.3. Screened plasmids expressions in the CFPS system

Fig.4. Relative activities of enzymes
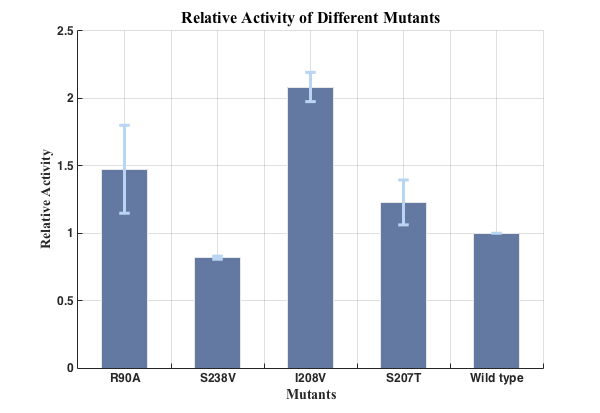
Fig.5. Relative activities of enzymes
Summary
We successfully screened two mutants (R90A &I208V) with higher enzyme activity by site-directed mutation.


