HomePage • PARTS • Basic Part
Basic Parts
Favorite Basic Part
PPI fluorescent reporter based on split-GFP
Since protein-protein interactions (PPIs) have been reported to play important roles in signal transduction and gene expression, methods for monitoring PPIs in cells have been developed rapidly for years1. In our registered and submitted parts, we provide two split-GFP fluorescence reporter systems, split-HRP enzymatic-based reporter systems and split Luciferase chemiluminescence-based reporter system.
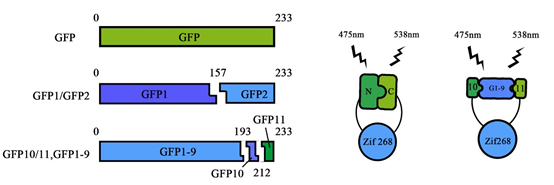
Our favorite basic part BBa_K1997014 is the core of the newly registered split-GFP reporter system as well as an important member of the collection PPI tool kit. Its function was based on tripartite association between two 20 amino-acids long GFP tags, GFP10 and GFP11 respectively, and the complementary GFP1-9 detector. When proteins interact, GFP10 and GFP11 self-associate with GFP1-9 to reconstitute a functional GFP (Figure 1).
Figure 1. Schematic representation of different approaches for split-GFP. Shown also were the method we stimulate the PPI in our experiments.
TO PROOF THE FUNTION of this part, a composite part (BBa_K1997018) with the addition of lac promoter, RBS and double terminator was constructed. After overnight expressed in E.coli, relative fluorescence intensity was calculated (Figure 2).
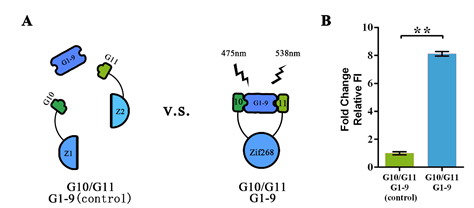
Figure 2. Evaluation of the Signal-Noise Ratio of split GFP system.
(A) Schematic representation of the evaluation protocol. The complete Zif-268 protein was introduced to simulate the condition where strong interaction among two proteins occur, whereas the split-zif protein was used to simulate the condition where no interaction exists. (B) Fluorescent assay showing the fluorescent intensity under two different conditions. Relative FI was calculated with normalization of the OD600 value. This experiment was run in three parallel reactions, and the data represent results obtained from at least three independent experiments. **p<0.01.
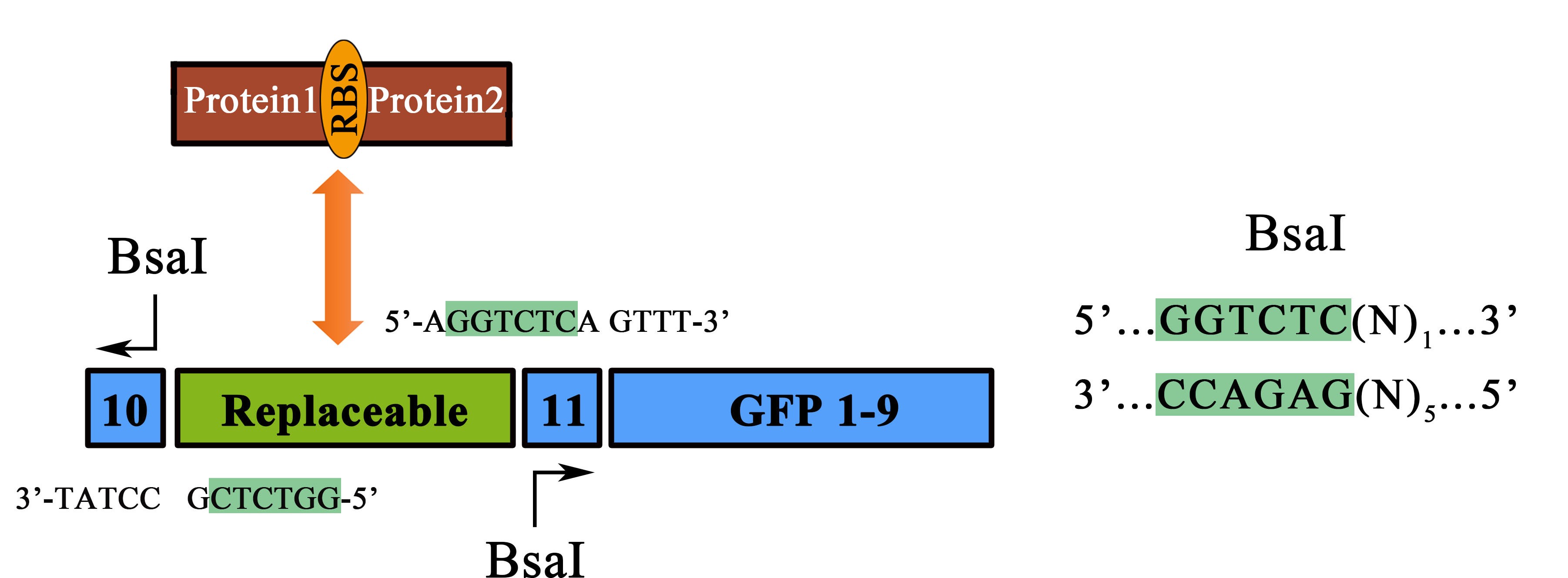
TO OPTIMIZE THE APPLICABILITY AND ADAPTIVE of this system for protein-protein interaction usage, we designed a novel substitution system for this part as well as for all the collection PPI tool kit. Following this, two proteins could be fused with their corresponding split-GFP fragment at the same time using Bsa I enzyme digestion and Golden-Gate Assembly protocols. For instance, the replaceable Zif268 segment can be replaced by other Protein1-RBS-Protein2 fragment, thus, whether the protein1 and 2 are interaction proteins could be determined by fluorescence signal (Figure 3)..
Figure 3 Schematic representation of the workflow of the substitution system
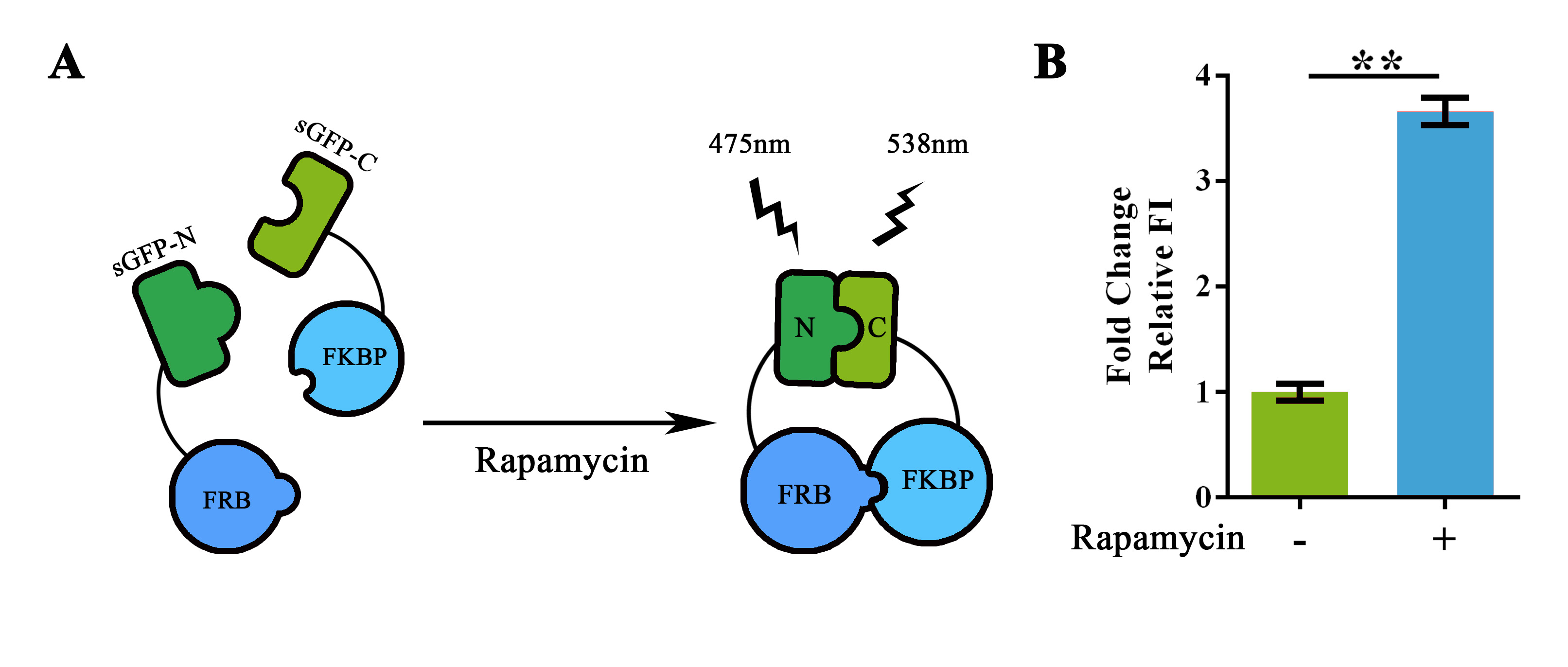
TO DEMONSTRATE the usability of this substitution system, we introduced a “FRB-RBS-FKBP” fragment to replace the replaceable fragment of this part by using Golden-Gate Assembly (BBa_K1997020). Intriguingly, IT WORKED! Fluorescence intensity measured right after the addition of Rapamycin showed a significant improvement on relative FI, thus validated the usability of this part and the substitution system (Figure 4).
Figure 4. Rapamycin-induced sGFP-N-FRB/sGFP-C-FKBP interaction.
(A) Schematic representation of the rapamycin induced protein-protein interaction. The adding of rapamycin would induce the interaction between FRB and FKBP, thus shortened the range between split-GFP fragments and reconstruct its structure for fluorescence generation. (B) Fluorescent assay showing the fluorescent intensity with/without Rapamycin induction. Relative FI was calculated with normalization of the OD600 value. For Fold change Relative FI, relative FI of the group without Rapamycin induction was set arbitrarily as 1.0, and the levels of the other groups were adjusted correspondingly. The concentration of Rapamycin used in the experiment was 40nM. This experiment was run in three parallel reactions, and the data represent results obtained from at least three independent experiments. **p<0.01.
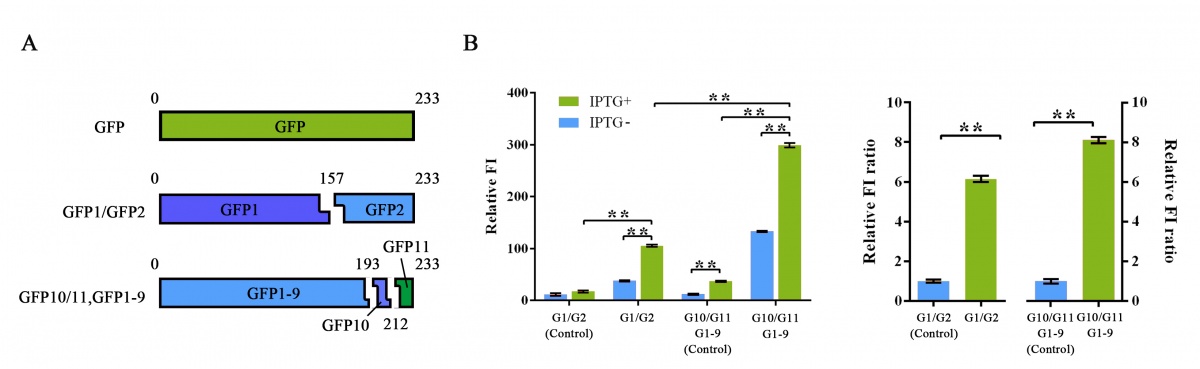
TO COMPARE this part related newly-introduced split-GFP system with the traditional GFP-N/GFP-C split-GFP system, we analyzed their SIGNAL INTENSITIES and the NOISE-SIGNAL RATIOS. After overnight expressed in E.coli, Relative fluorescence intensity was calculated and higher signal intensity and NSR was shown on the newly-introduced system (Figure 5).
Figure 5 Evaluation of two different split-GFP systems.
Relative fluorescence intensity was calculated with normalization of the OD600 value. For Relative FI ratios, relative fluorescence intensity of each control group was set arbitrarily at 1.0, and the levels of the other groups were adjusted correspondingly. This experiment was run in three parallel reactions, and the data represent results obtained from at least three independent experiments. *p<0.05, **p<0.01.
Collectively, this is an elaborate part for an optimal split-GFP reporting system, which was easy to be edit as a functional protein-protein interaction system.
Our Basic Parts
| NAME | TYPE | DISCRIPTION | LENGTH | SUBMITTED |
| BBa_K1997000 | Coding | sHRP-N | 702 | yes |
| BBa_K1997001 | Coding | sHRP-C | 345 | yes |
| BBa_K1997002 | Coding | sluc-N | 1308 | yes |
| BBa_K1997003 | Coding | sluc-C | 519 | yes |
| BBa_K1997004 | Reporter | sGFP-N->Zif268->sGFP-C | 1062 | yes |
| BBa_K1997005 | Reporter | sHRP-N->FRB->RBS->FKBP-> sHRP-C | 1653 | yes |
| BBa_K1997006 | RNA | let-7a | 87 | yes |
| BBa_K1997007 | RNA | let-7e | 87 | yes |
| BBa_K1997008 | RNA | let-7f | 87 | yes |
| BBa_K1997009 | RNA | let-7g | 87 | yes |
| BBa_K19970010 | Coding | dCas9 | 4104 | yes |
| BBa_K19970014 | Reporter | GFP10->Zif268->GFP11->RBS->GFP1-9 | 1122 | yes |