Bronze
We created 7 new BioBricks for bronze medal criterion. Document 7 new standard BioBrick Parts to our project and submit these parts to the iGEM Registry.
We created 7 new BioBricks for bronze medal criterion. Document 7 new standard BioBrick Parts to our project and submit these parts to the iGEM Registry.
| Parts | Biobrick-Number | Length [bp] | Designer |
| vp64 | BBa_K1982012 | 246 | NEU-China 2016 |
| Prokaryotic tCAS9 | BBa_K1982001 | 4122 | NEU-China 2016 |
| Prokaryotic CRY2( a blue light stimulated photoreceptor) | BBa_K1982002 | 1854 | NEU-China 2016 |
| CIBN(the N-terminal fragment of CIB1) | BBa_K1982003 | 612 | NEU-China 2016 |
| Eukaryotic tCAS9 | BBa_K1982007 | 4121 | NEU-China 2016 |
| Eukaryotic CRY2 ( a blue light stimulated photoreceptor) | BBa_K1982009 | 1848 | NEU-China 2016 |
| tCas9-CIBN (Prokaryotic LACE system) | BBa_K1982000 | 4731 | NEU-China 2016 |
Silver
We created 5 new BioBrick devices for silver medal criterion.
| Parts | Biobrick Number | Length [bp] | Designer |
| tCas9-Vp64(Prokaryotic) | BBa_K1982006 | 4368 | NEU-China 2016 |
| CRY2-VP64(Prokaryotic LACE system) | BBa_K1982005 | 2100 | NEU-China 2016 |
| tCas9-CIBN (Eukaryotic LACE system) | BBa_K1982008 | 4731 | NEU-China 2016 |
| CRY2-VP64(Eukaryotic LACE system) | BBa_K1982010 | 2100 | NEU-China 2016 |
| tCas9-Vp64(Eukaryoticc) | BBa_K1982011 | 4368 | NEU-China 2016 |
Gold
We also Improved a previous iGEM project
We improved CRY2 (BBa_K1592015) and CIB1 (BBa_K1592016). These biobricks are key BioBrics in " A live eukaryotic cell based auto-cementation kit " project of HUST-China 2015. CIB1 encodes a basic helix-loop-helix (bHLH) protein, would interact with cryptochrome 2 (CRY2), a blue light stimulated photoreceptor. This fusion protein is for use in a yeast-two-hybrid system, and a Gal4 DNA activating domain fused to N terminus of CIB1. To regulate DNA transcription by blue light, the system is based on a two-hybrid interaction in which a light-mediated protein interaction brings together two halves (a binding domain and an activation domain) of a split transcription factor.
We improved the function of CRY2 (BBa_K1592015) and CIB1 (BBa_K1592016) and enter our parts (BBa_K1982005 and BBa_K1982000) in the Registry. Please see the Registry page of this two parts.
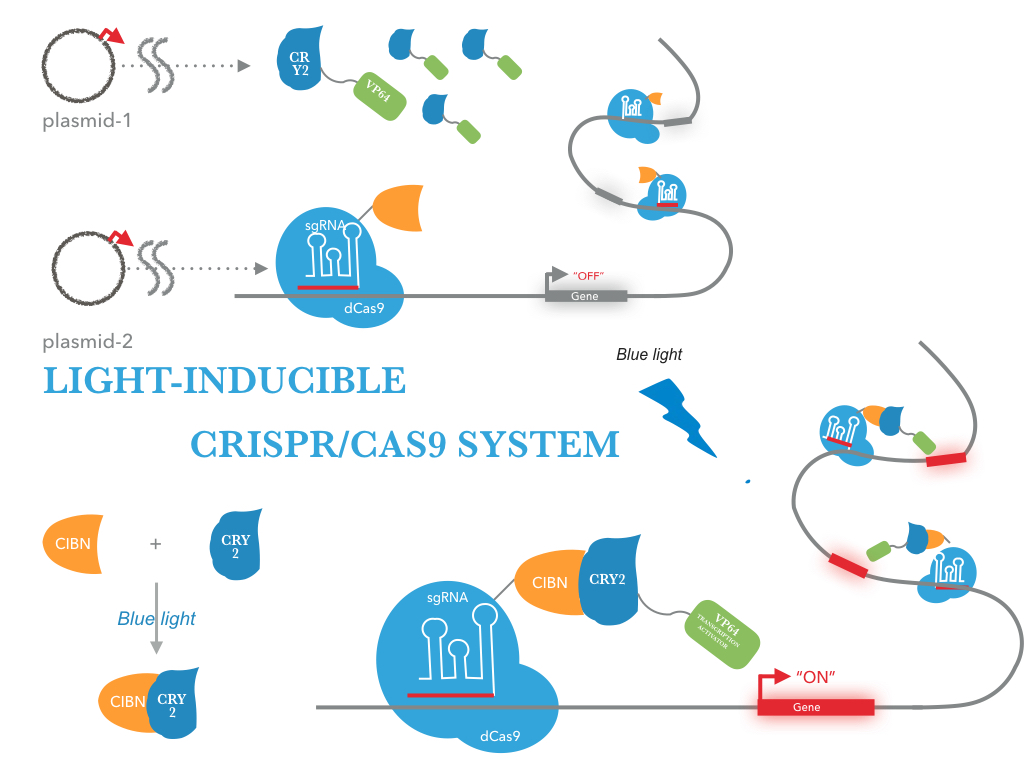
Figure 1 illustrates the detailed design of the whole device
Proof of concept
According to our design, we transfected a targeting plasmid into E.coli BL21 to express fusion protein composed of the protein tCas9 and CIBN. Simultaneously, we transfected GFP, gRNA and CRY2-VP64 plasmid into E.coli BL21. Then, the promoter of GFP were continually repressed by gRNA and tCas9. When exposed to blue light, the N-terminal fragment of CIB1 (CIBN) would interact with CRY2. Subsequently, binding of the CRY2-VP64 on the upstream sequence of promoter will result in activating promoter via recruitment of the RNA polymerase II by the VP64 subunit of CRY2-VP64. Generally, the experimental procedure can be divided into four steps:
- Characterization of fusion protein
- Verification of the suppression efficiency of gRNA
- Silencing capability validation
- Targeting capability validation
Characterization of fusion protein

Figure 1: Validation of protein
CRY2-VP64:
Stationary cultures of BL21 J23114 was subcultured into fresh media and growth for 4 hours. Subsequent extraction of protein from bacterial and visualization using SDS-PAGE confirms that proteins of the expected size are present in the supernatant and hence most likely successfully secreted by the engineered bacterial strains.

Figure 2:Western blot analysis of CRY2-VP64 protein levels.
1:Control groups transfected with empty plasmid; 2 3 4:Transfected with CRY2-VP64 plasmid (J23114 promoter)
from different single colonies, ~78.5 kDa. Stationary cultures of BL21 was subcultured into fresh media and growth for
4 hours. 30ng of protein with total volume of 30ul (protein sample + dissociation buffer).
tCas9-CIBN:
Stationary cultures of BL21 pBAD was subcultured into fresh media and induced for 4 hours or 16 hours using different concentrations L-arabinose. Subsequent purification of protein from the cell-free supernatant and visualization using SDS-PAGE confirms that proteins of the expected size are present in the supernatant and hence most likely successfully secreted by the engineered bacterial strains.

Figure 3:Western blot analysis of tCas9-CIBN protein levels.
1 3 5 7 9 11:Control groups transfected with empty plasmid; 2 4 6 8 10 12: Transfected with tCas9-CIBN plasmid
(pBAD promoter), ~180 kDa
. Stationary cultures of BL21 was subcultured into fresh media and growth for 4 hours or 16 hours using different concentrations L-arabinose. 30ng of protein with total volume of 30ul (protein sample + dissociation buffer).

Figure 4: Western blot analysis of tCas9-CIBN protein levels.
1 3 5 7 9 11:Control groups transfected with empty plasmid; 2 4 6 8 10 12: Transfected with tCas9-CIBN plasmid
(pBAD promoter), ~180 kDa
. Stationary cultures of BL21 was subcultured into fresh media and growth for 4 hours or 16 hours using different concentrations L-arabinose. 30ng of protein with total volume of 30ul (protein sample + dissociation buffer).
Verification of the suppression efficiency of gRNA
With different target loci have been tested by the usage of a GFP reporter plasmid (pCold-1) with a CSPA promotor. The target sites can be determined by directing the gRNA consisting of 20 bp length against the desired sequence of interest. F1 gRNA with target sites at different distances to the promotor regions proved successfully as potential activation sites (see Table 1 and Figure 5).
| Name | Binding Site | Distance to promoter | Sequence | Position |
| F1 | CSPA promoter | 15 | TGCATCACCCGCCAATGCG | sense sequences |
| F2 | non-coding | 68 | GCCGCCGCAAGGAATGGTG | sense sequences |
| R1 | CSPA promoter | 43 | ATTAATCATAAATATGAAA | antisense sequences |
| R2 | non-coding | 94 | CATCATCCAACTCCGGCAAC | antisense sequences |
Table 1: Overview of the tested gRNAs with different binding sites on the GFP pCold-1 plasmid.

Figure 5: Position of the target loci on the GFP pCold-1 plasmid.
To ensure the suppression efficiency of the gRNA, four gRNA sequences targeting different sites of CSPA promoter were designed and transfected into the E. coli strains BL21. Efficient suppression of CSPA promoter in strains BL21 was observed. GFP levels in GFP transgenic strains decrease after inserting a fragment that expresses CSPA promoter gRNA. And the sequence with the best suppression effect was selected for further study.


Figure 6: Results of the GFP-influence under the CSPA promotor
only using different gRNAs targeted to CSPA promoter in BL21.
1-8: Transfected with different GFP-gRNA plasmid (CSPA promoter) 9:Control group transfected with GFP plasmid,~27 kDa. Excitation wavelength: 488 nm, Emission wavelength: 509nm. Stationary cultures of BL21 was subcultured into fresh media and growth for 8 hours (1 3 5 7 9) or 16 hours (2 4 6 8) at 30°C. 30ng of protein with total volume of 30ul (protein sample + dissociation buffer).
Silencing capability validation
We next evaluated the effect of tCas9-cibn on suppressing CSPA promoter. GFP expression levels were assayed in strains BL21 after co-transformation with tCas9-cibn and gRNA.


Figure 7: Silencing capability of tCas9-CIBN with gRNA
using different
gRNAs targeted to CSPA promoter in BL21.
1-8: Transfected with different GFP-gRNA plasmid (CSPA promoter) and tCas9-CIBN plasmid (pBAD promoter)
9 10:Control groups transfected with GFP plasmid and tCas9-CIBN plasmid (pBAD promoter), ~27 kDa. Excitation wavelength: 488 nm, Emission wavelength: 509nm. Stationary cultures of BL21 was subcultured into fresh media and growth for 8 hours (1 3 5 7 9) or 16 hours (2 4 6 8 10) using 15mM L-arabinose at 30°C. 30ng of protein with total volume of 30ul (protein sample + dissociation buffer).
Compared with control groups, green fluorescence intensity and mRNA levels were dramatically reduced in groups treated with gRNA and tCas9-CIBN. These results suggest that gRNA can specifically guide tCas9 to target upstream of CSPA promoter, thereby to inhibit CSPA promoter to reduce GFP expression levels.
Activation capability validation
Spatially controlled activation of gene expression was achieved in strains co-transfected with the LACE system, a reporter vector containing a gRNA target sequence upstream of CSPA promoter and the eGFP gene. Strains transfected with light-activated CRISPR/Cas9 effector (LACE) and incubated in the dark did not show a significant difference in eGFP levels compared to control groups transfected with empty plasmid. Strains containing the LACE system and gRNA exhibited significantly brighter eGFP fluorescence intensity when illuminated compared to when incubated in the dark. Activation of the eGFP reporter in strains transfected with the gRNA and LACE constructs, the gRNA and tCas9-VP64 expression plasmid or an empty plasmid as a negative control was quantified after 24 hours of illumination or incubation in the dark.

Figure 8: Activation capability of LACE system
using gRNA-F1 plasmid and tCas9-CIBN plasmid in BL21.
Excitation wavelength: 488 nm, Emission wavelength: 509nm. Stationary cultures of BL21 was subcultured into fresh media and growth for 8 hours using 15mM L-arabinose at 30°C.
Conclusion
The LACE system provides a straightforward and robust optogenetic method to regulate the expression of endogenous genes using the CRISPR/tCas9 system. When co-transfected with gRNAs into E.coli that are stimulated with blue light, LACE produces a high level of transcriptional activation, in some cases, comparable to those observed with tCas9-VP64.



















