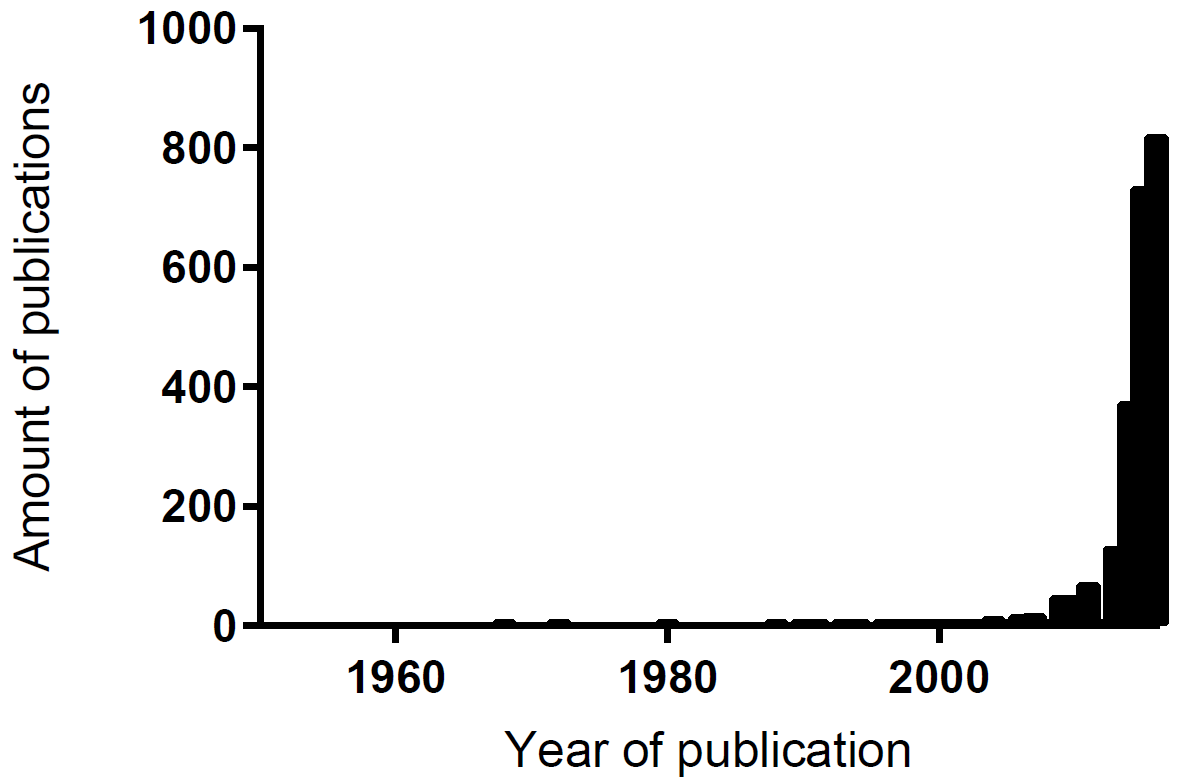
Bioprinting
An emerging path that could revolutionize science
3D bioprinting comes up with some challenges that are not addressed in non-tissue printing: choice of cell types, assembly method, growth, differentiation and vascularization factors as well as technical difficulties concerning the sensitivity of the biological material. Bioprinting is, therefore, a melting pot of natural sciences and thus a highly interdisciplinary field. Due to its high complexity and tremendous relevance for synthetic biology, we even recommend and advocate a future iGEM-track only for bioprinting; the imagination of new possibilities offered by overall functional and fully controllable bioprinting is sheer endless. Our self-assembled bioprinter makes further improvements by future iGEM-teams possible. Following short description should reveal an idea about the variety of 3D-bioprinters:
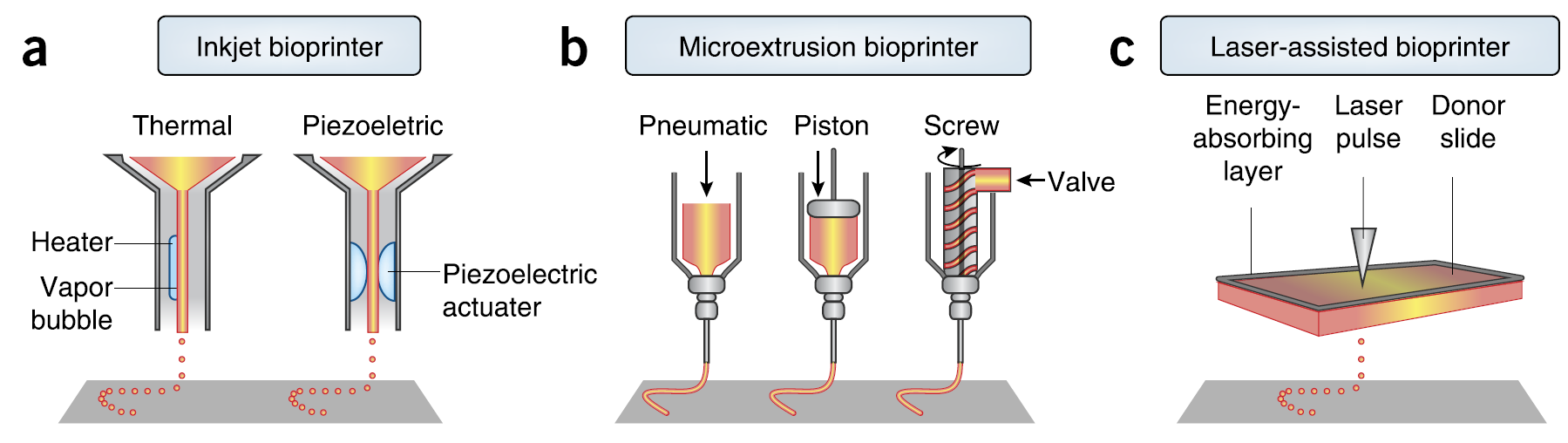
In general, there are three types of bioprinters: inkjet, microextrusion, and laser-assisted printers. Inkjet bioprinters are working either on a thermal basis using electricity to heat the printhead that leads to air pressure pulses[1]. The created force lets droplets flow out of the nozzle in a controlled manner. Or inkjet bioprinters are based on acoustic waves produced by piezoelectric or ultrasound pressure. In contrast to gradually working inkjet printers, microextrusion bioprinters have pneumatic or mechanical (like ours) dispensing systems that are capable of continuously extruding biomaterials. Laser-based bioprinters are also delivering cells only gradually by focusing pressure on cell-containing slides leading to their drop-off on a collector substrate[2].[3]
These techniques differ mainly in three factors: surface resolution, cell viability, and used biomaterials. In our case, the latter part is redundant, since we print directly cells without a certain scaffold[4].
| Inkjet | Microextrusion | Our bioprinter | |
| Cell viability | >85% | 40-80% | According to our calculations near 100% |
| Material viscosities | 3.5-12 mPa/s | 30 mPa/s to >6 x 107 mPa/s | 1.5*10-3 |
| Gelation methods | Chemical, photo-crosslinking | Chemical, photo-crosslinking | not needed |
| Print speed | Fast (1-10000 droplets/s) | Slow (10-20 µm/s) | Slow |
| Resolution or droplet size | <1 pl to >300 pl droplets, 50 µm wide | 5 µm to millimeters wide | 20 µm (depends on nozzle size) |
| Cell densities | Low, <106 cells/ml | High, cell spheroids | <108 |
| Printer cost | Low | Medium | Very low (<2000€) |
It's all about the ink
Besides the multiple variants of available bioprinters there are also many diffrent so called Bioinks. Nowadays they're based on two main principles: matrix and scaffold.
Using a matrix means in general printing with a hydrogel. This is composed of highly hygroscopic material that, after gelation upon a certain stimulus, forms a gel-like structure that holds the cells in their position and provides the diffusion of nutrients and oxygen. Since these matrices must be biocompatible, natural materials such as collagen, spider-silk[7], fibrin or alginate are often used. They differ in the speed of polymerization as well as in the stimulus that induces gelation (e.g. thermal or chemical).[8]
The greatest disadvantage of those techniques is that the polymerization process still takes several minutes, which is time that you don't have when printing the cells in a precise 3-dimensional manner. Alternative processes that are faster, such as photocrosslinking, are due to the emergence of free radicals and the radiation often harmful to the cells.
With our biotINK based on recombinant membrane proteins and streptavidin we killed two birds with one stone: 1) No time loss: Polymerization takes place just upon printing due to the fast and specific interaction between biotin and streptavidin 2) No chemicals, high temperatures or radiation needed: All components needed to stick the cells together are an intrinsic and 100% biocompatible part of the system.
References
- ↑ Cui, X., Boland, T., D D'Lima, D., & K Lotz, M. (2012). Thermal inkjet printing in tissue engineering and regenerative medicine. Recent patents on drug delivery & formulation, 6(2), 149-155.
- ↑ Barron, J. A., Wu, P., Ladouceur, H. D., & Ringeisen, B. R. (2004). Biological laser printing: a novel technique for creating heterogeneous 3-dimensional cell patterns. Biomedical microdevices, 6(2), 139-147.
- ↑ Murphy, S. V., & Atala, A. (2014). 3D bioprinting of tissues and organs. Nature biotechnology, 32(8), 773-785.
- ↑ Xu, T., Zhao, W., Zhu, J. M., Albanna, M. Z., Yoo, J. J., & Atala, A. (2013). Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials, 34(1), 130-139.
- ↑ Murphy, S. V., & Atala, A. (2014). 3D bioprinting of tissues and organs. Nature biotechnology, 32(8), 773-785.
- ↑ Murphy, S. V., & Atala, A. (2014). 3D bioprinting of tissues and organs. Nature biotechnology, 32(8), 773-785.
- ↑ Schacht, K., Jüngst, T., Schweinlin, M., Ewald, A., Groll, J., & Scheibel, T. (2015). Biofabrication of Cell‐Loaded 3D Spider Silk Constructs. Angewandte Chemie International Edition, 54(9), 2816-2820.
- ↑ Nicodemus, G. D., & Bryant, S. J. (2008). Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Engineering Part B: Reviews, 14(2), 149-165.