(→Mass spectroscopy) |
(→Fluorescence spectroscopy) |
||
| Line 28: | Line 28: | ||
===Fluorescence spectroscopy=== | ===Fluorescence spectroscopy=== | ||
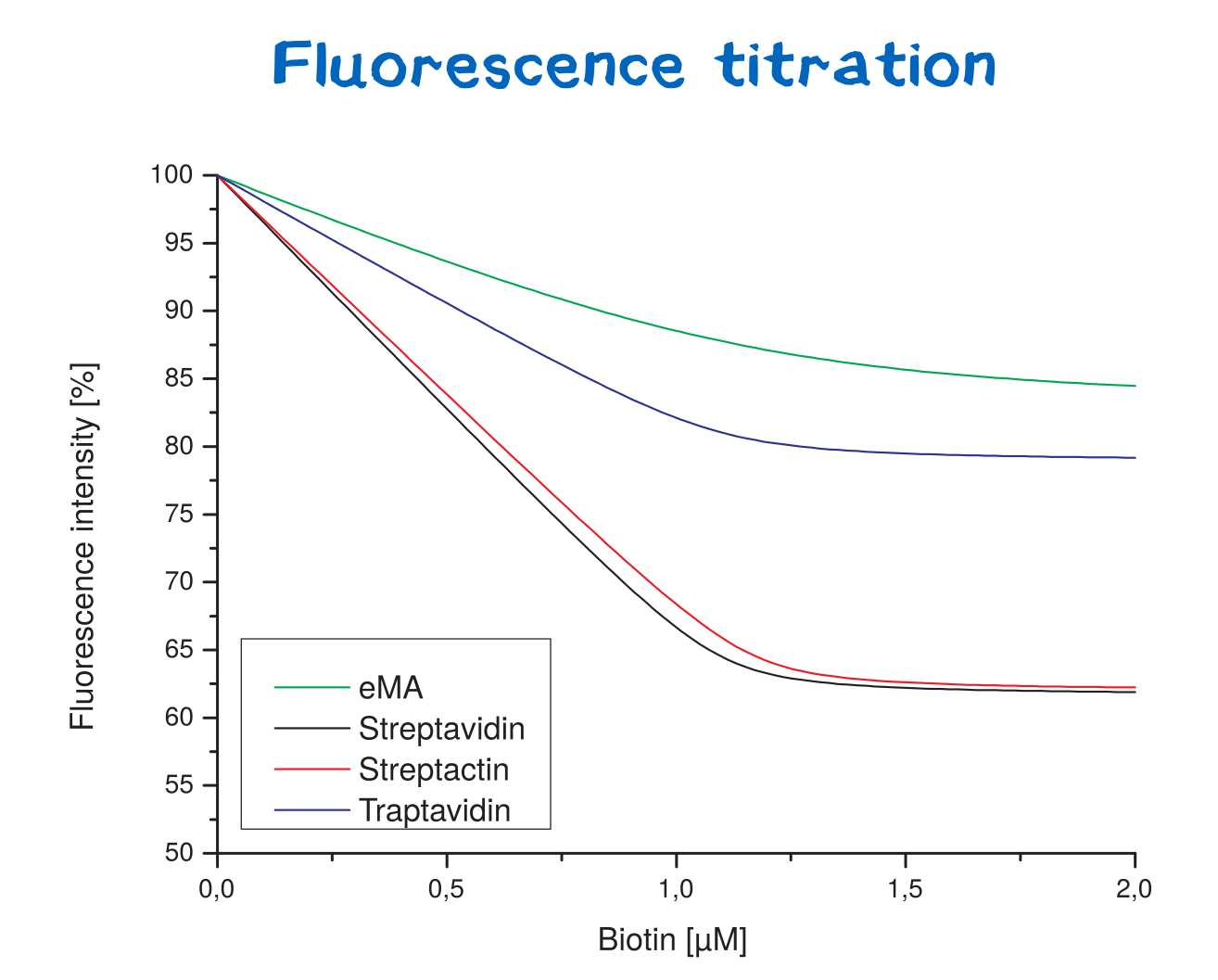
| + | [[File:Muc16_results_flt.png|thumb|right|250px| <i><b>Figure 3:</b></i> Fluorescence titration curves for various avidin variants.]] | ||
| + | |||
===Surface plasmon resonance=== | ===Surface plasmon resonance=== | ||
Unfortunately every single Avidin variant bound on the surface of the used chip. Therefore no evaluable results were gathered. | Unfortunately every single Avidin variant bound on the surface of the used chip. Therefore no evaluable results were gathered. | ||
Revision as of 12:21, 19 October 2016
Characterization of various Avidin variants
Streptavidin and its variants were produced by cytoplasmic expression in E. coli in inclusion bodies. After stabilization and refolding of the functional tetrameric and monomeric variants, purification was carried out by ammonium sulfate precipitation, Ion Exchange Chromatography and size exclusion chromatography. Samples of each required step were analysed by SDS-PAGE. Finally each of the successfully purified variants were characterized. Mass spectrometry was used to confirm the expected size, circular dichroism spectroscopy (cd) to determine folding states, fluorescence titration and surface plasmon resonance for determining binding affinities.
Results can be seen in Table 1.
Mass spectroscopy
To determine if the mass of each measured protein conforms to our expectations based on the sequence, mass spectra were recorded and can be seen in the following figure 1-3.
Circular dichroism spectroscopy
De- and renaturation curves as well as UV-CD-spectra were measured and can be seen in figure 1 and 2. All cd spectra were evaluated by Spectra Manager Software and results can be seen in figure 3. Also the temperature where 50 % of the protein were unfolded were calculated. Therefore the data for each protein were entered into a GraphPad Prism sheet and calculated respectively. Results can be seen in table 1.
Fluorescence spectroscopy
Surface plasmon resonance
Unfortunately every single Avidin variant bound on the surface of the used chip. Therefore no evaluable results were gathered.
| protein | amino acids (without Met) | molecular weight monomer [Da] | molecular weight tetramer [Da] | theoretical isoelectric proint (pI) | pI (Isoelectric focusing) | extinction coefficient (monomer, ε280) [M-1 cm-1] | KD |
|---|---|---|---|---|---|---|---|
| Streptavidin wt | 126 | 13200.34 | 52801.36 | 6.09 | 41940 | ||
| Traptavidin | 126 | 13129.22 | 52516.88 | 5.14 | 41940 | ||
| Streptactin | 126 | 13241.44 | 52516.88 | 8.32 | 41940 | ||
| enhanced monomeric Avidin | 138 | 15220.30 | / | 5.91 | 35075 |
Kopiervorlagen
Seitenverantwortliche/r: Wird am Schluss gemacht.
Literaturreferenz
Literaturreferenz[1]
Bei Google Scholar bitte das APA-Ziteirformat verwenden.
Textformatierung
kursiv
fett
Strich
Links
Wikiinterner Link Team:LMU-TUM_Munich/Materials (As described in the Materials section)
Wikiexterner Link Visit W3Schools
Visit W3Schools
Bilder
Introduction
Design
Experiments
Proof of concept
Demonstrate
Discussion
References
- ↑ Schmidt, T. G., & Skerra, A. (2007). The Strep-tag system for one-step purification and high-affinity detection or capturing of proteins. Nature protocols, 2(6), 1528-1535.