VolkerMorath (Talk | contribs) |
(→Abstract: biotINK - rethINK tissue printing) |
||
| Line 19: | Line 19: | ||
==<span style="color:#000000">Abstract:</span> <span style="color:#009440">biot</span><span style="color:#3070b3">INK</span> <span style="color:#8d8d8d">- rethINK tissue printing</span>== | ==<span style="color:#000000">Abstract:</span> <span style="color:#009440">biot</span><span style="color:#3070b3">INK</span> <span style="color:#8d8d8d">- rethINK tissue printing</span>== | ||
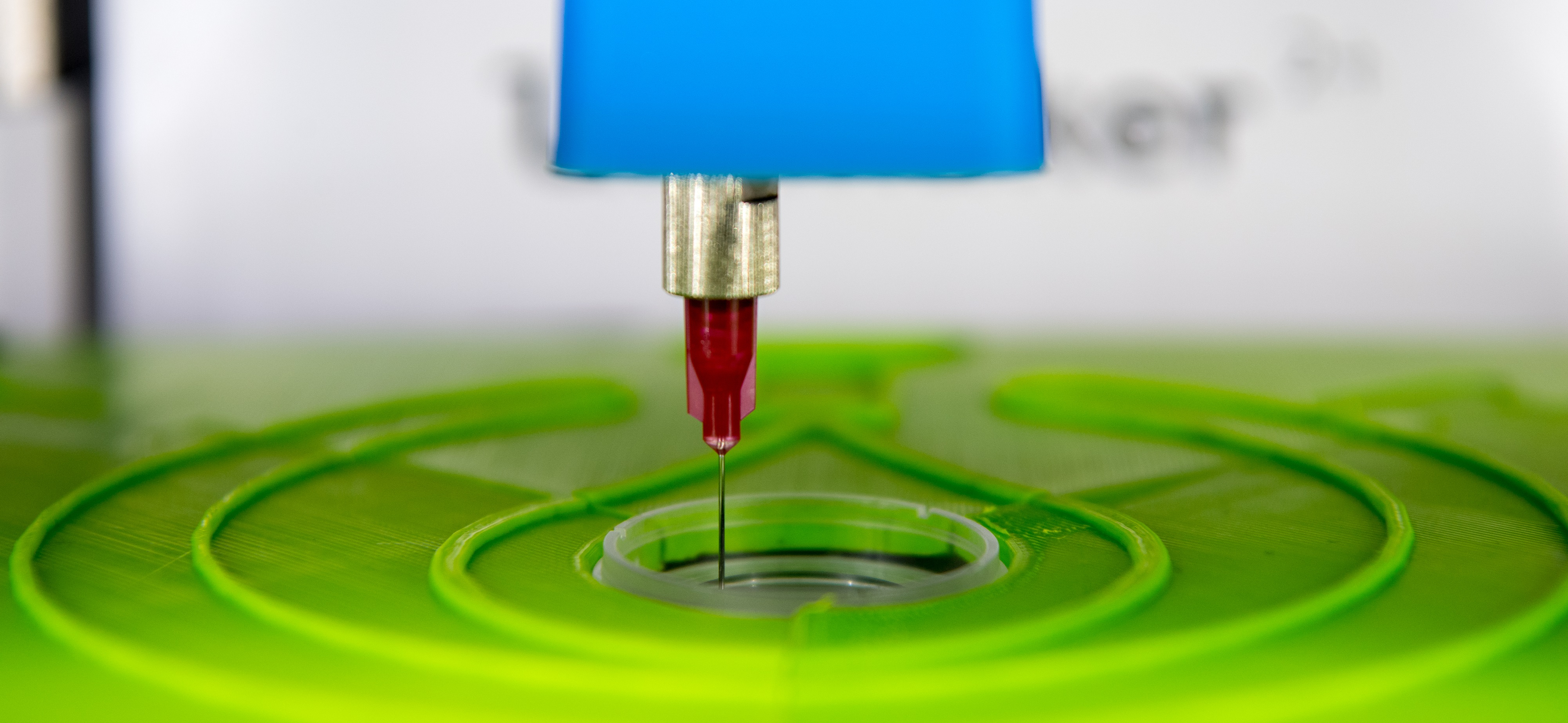
| − | We are living in an aging society that is facing a decreasing supply of donor organs. To confront this pressing issue, we developed a game-changing approach to bioprint tissues for biomedical applications. Our interdisciplinary work aims to create a unique ink, named biotINK, to revolutionize bioprinting. The printing process uses a [https://2016.igem.org/Team:LMU-TUM_Munich/Hardware hijacked 3D printer] and two components of biotINK to induce an instantaneous [https://2016.igem.org/Team:LMU-TUM_Munich/Proof polymerization reaction], creating three-dimensional multi-cellular structures in a user-definable manner. The principle of this two-component glue relies on the rapid and specific interaction of biotin and its tetrameric [https://2016.igem.org/Team:LMU-TUM_Munich/Proteins binding protein] | + | We are living in an aging society that is facing a decreasing supply of donor organs. To confront this pressing issue, we developed a game-changing approach to bioprint tissues for biomedical applications. Our interdisciplinary work aims to create a unique ink, named biotINK, to revolutionize bioprinting. The printing process uses a [https://2016.igem.org/Team:LMU-TUM_Munich/Hardware hijacked 3D printer] and two components of biotINK to induce an instantaneous [https://2016.igem.org/Team:LMU-TUM_Munich/Proof polymerization reaction], creating three-dimensional multi-cellular structures in a user-definable manner. The principle of this two-component glue relies on the rapid and specific interaction of biotin and its tetrameric [https://2016.igem.org/Team:LMU-TUM_Munich/Proteins binding protein] avidin. To make use of this high biotin-avidin affinity for cell-cell cross-linking, we [https://2016.igem.org/Team:LMU-TUM_Munich/Receptors engineered cells presenting biotin moieties or biotin-binding proteins on their surfaces] as well as [https://2016.igem.org/Team:LMU-TUM_Munich/Proteins recombinant matrix proteins], which [https://2016.igem.org/Team:LMU-TUM_Munich/Proof co-polymerize upon printing]. Furthermore, we explored different genetic circuits which allow us to functionalize the bio-synthetic tissue and install biosafety mechanisms. <br> |
Altogether, we are confident that our system provides the necessary means to advance the SynBio community to the next level – the tissue level.<br> | Altogether, we are confident that our system provides the necessary means to advance the SynBio community to the next level – the tissue level.<br> | ||
[[File:Muc_Finalsphoto_001.png|center|950px]] | [[File:Muc_Finalsphoto_001.png|center|950px]] | ||
Revision as of 11:55, 18 November 2016
2016/11/02: We will update our wiki within the next 10 days. Please do not hesitate to contact us at iGEM2016.Munich@gmail.com.
Abstract: biotINK - rethINK tissue printing
We are living in an aging society that is facing a decreasing supply of donor organs. To confront this pressing issue, we developed a game-changing approach to bioprint tissues for biomedical applications. Our interdisciplinary work aims to create a unique ink, named biotINK, to revolutionize bioprinting. The printing process uses a hijacked 3D printer and two components of biotINK to induce an instantaneous polymerization reaction, creating three-dimensional multi-cellular structures in a user-definable manner. The principle of this two-component glue relies on the rapid and specific interaction of biotin and its tetrameric binding protein avidin. To make use of this high biotin-avidin affinity for cell-cell cross-linking, we engineered cells presenting biotin moieties or biotin-binding proteins on their surfaces as well as recombinant matrix proteins, which co-polymerize upon printing. Furthermore, we explored different genetic circuits which allow us to functionalize the bio-synthetic tissue and install biosafety mechanisms.
Altogether, we are confident that our system provides the necessary means to advance the SynBio community to the next level – the tissue level.
Sponsors