(→Constructs and methodology) |
(→Results) |
||
| Line 73: | Line 73: | ||
| − | '''Two interesting | + | '''Two interesting conclusions''' could be drawn from the results of the luciferase assays and the ELISA. |
Revision as of 01:34, 19 October 2016
Fighting hypoxia in printed tissue
Unicellular organisms such as bacteria posses the ability to reproduce very quickly when given the right conditions such as warmth, moisture and suitable nutrients,[1] and enter an inactive state when these are limited. On the contrary, most multicellular systems depend on a steady supply of oxygen and nutrients essential for their survival and metabolic stability.
Herein lies one of the main challenges of bioprinting approaches. When printing, the homogenous delivery of oxygen to three-dimensional cell-constructs is limited by diffusion gradients in tissue from the periphery towards the center [2], thus resulting in tissue hypoxia. [3]
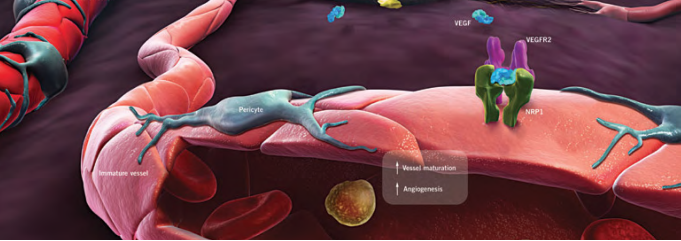
In our bodies, this need for oxygen supply is met by blood vessels, which form extensive networks that nurture all tissues. As a result, capillaries grow and regress in healthy tissues according to functional demands. Thereby, no metabolically active tissue in the body is more than a few hundred micrometers from a blood capillary, which is formed by the process of angiogenesis. [4]
Sprouting angiogenesis, as implied by its name is characterized by sprouts composed of endothelial cells, which usually grow toward an angiogenic stimulus.
This driving stimulus is initiated in poorly perfused tissues when oxygen sensing mechanisms detect a level of hypoxia that demands the formation of new blood vessels to satisfy the metabolic requirements of parenchymal cells. Most types of parenchymal cells respond to a hypoxic environment by secreting a key proangiogenic growth factor called vascular endothelial growth factor (VEGF-A). [5]
Because the production of a normal vasculature is among others heavily dependent upon the concentration of VEGF-A in the tissue, we have constructed a model in order to measure the hypoxia dependent secretion of VEGF and PDGF (the so-called platelet-derived growth factor). Thus, we may simulate the extreme conditions that are present when printing several layers of tissue and, by triggering sprouting angiogenesis, induce growth of blood vessels towards portions of tissues previously devoid of them.
Hypoxia-inducible promoters
(Hypoxia refers to subnormal levels of oxygen in air, blood and tissue. Tissue hypoxia leads to cellular dysfunction and can ultimately lead to cell death. The ability of cells to adapt to periods of hypoxia is therefore important for their survival. [6]) In this context, we wanted to find the most suitable way to compensate the poor oxygen conditions that are generated when printing tissue structures with a volume of more than a few mm3.
Following a previously published idea by Lee, J. Y., et al. "A novel chimeric promoter that is highly responsive to hypoxia and metals." to enhance the level of hypoxic induction and to control target gene expression [7], we have designed a potent hypoxia-inducible promoter system, making use of so-called hypoxia-response elements.
Many hypoxia-responsive genes are regulated by the hypoxia-response element (HRE) protein family of transcription factors, including hypoxia-inducible factor-1 (HIF-1). [8] When HREs derived from different genes are placed in plasmids, they confer hypoxia inducibility upon heterologous promoters in various cell types. [9] WE intend to use this system of hypoxia-inducible expression to induce vascularization in printed tissues.
Constructs and methodology
In order to test the responsivity of HREs, we incorporated different copy numbers (1x-HRE, 6x-HRE, 8x-HRE) upstream from a minimal promoter. The evaluation of the constructs' inductivity was performed by measuring the secretion of a luciferase reporter after transient transfection in HEK293T cells.
Parallely we expressed a HRE´s activated construct containing the actual growth factors VEGF and PDGF under the same conditions and compared it to a positive control consisting of a CMV driven VEGF and PDGF gene.
For the experiments cells were subjected to normoxia (21% O2, 5% CO2, 74% N2) and to hypoxia (1% O2, 7% CO2, 92% N2) for 24 hours.
By taking samples of the medium after 0 h, 12 h and 24 h after the exposure of the cells to this conditions and measuring luciferase activity by a corresponding assay, we were able to quantify hypoxia-dependent gene expression. To be able to determine how far gene expression is responsive to hypoxia in comparison to normal oxygen concentrations, a control at normoxic conditions was furthermore run.
For the assay, a total of five constructs were tested, three of which were luciferase constructs for the quantification of expression levels and two of which were VEGF-constructs that aimed to confirm the expression of the actual growth factor by our cells, as well as acting as a negative control for the luciferase assay. The luciferase constructs all contained the hypoxia promoter.
Results
Results of the luciferase assay:
Results of the ELISA:
Two interesting conclusions could be drawn from the results of the luciferase assays and the ELISA.
- Although two of the nanoluciferase constructs did not seem to be functional (hypoxia promoter with 2x HRE and 4x HRE), a tendency could be observed for the others - showing that the number of HREs positively correlates with expression levels - the constructs with 6x and 8x HREs showing the highest expression/secretion levels. In order to determine whether the differences between the nanoluciferase constructs containing 6x HRE and 8x HRE are statistically significant, a two-tailed t-test was performed. Resulting P-values of 0.98 and 0.87 for 12 h values, as well as 0.74 and 0.52 for 24 h (hypoxia and normoxia, respectively) indicate that the populations by conventional definition do not significantly differ. This may hint at the fact that more than 6 HREs do not increase promoter activity either in presence or abscence of oxygen.
- Expression levels compared between hypoxia and normoxia do not significantly differ at both time points , making the promoter created by us not actually responsive towards states of low oxygen. High. Still, in comparison to the CMV promoter (as visible during the ELISA), the hypoxia promoter does not lose activity upon exposing cells to hypoxia, but remains remarkably steady. The HRE elements may hereby counter-act the effects of reduced transcription due to hypoxia-mediated stress signalling.
References
- ↑ http://www.bbc.co.uk/schools/gcsebitesize/science/triple_ocr_gateway/beyond_the_microscope/understanding_microbes/revision/2/
- ↑ Volkmer, Elias, et al. "Hypoxia in static and dynamic 3D culture systems for tissue engineering of bone." Tissue Engineering Part A 14.8 (2008): 1331-1340
- ↑ Höckel, Michael, and Peter Vaupel. "Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects." Journal of the National Cancer Institute 93.4 (2001): 266-276.
- ↑ Adair TH, Montani JP. Angiogenesis. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. Chapter 1, Overview of Angiogenesis doi:10.1089/ten.tea.2007.0231
- ↑ Adair TH, Montani JP. Angiogenesis. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. Chapter 1, Overview of Angiogenesis doi:10.1089/ten.tea.2007.0231
- ↑ Muz, Barbara, et al. "Hypoxia. The role of hypoxia and HIF-dependent signalling events in rheumatoid arthritis." Arthritis research & therapy 11.1 (2009): 1
- ↑ Lee, J. Y., et al. "A novel chimeric promoter that is highly responsive to hypoxia and metals." Gene therapy 13.10 (2006): 857-868
- ↑ Lee, J. Y., et al. "A novel chimeric promoter that is highly responsive to hypoxia and metals." Gene therapy 13.10 (2006): 857-868
- ↑ Boast K, Binley K, Iqball S, Price T, Spearman H, Kingsman S et al. Characterization of physiologically regulated vectors for the treatment of ischemic disease. Hum Gene Ther 1999; 10: 2197–2208