Fighting hypoxia in printed tissue
Unicellular organisms such as bacteria posses the ability to reproduce very quickly, when given the right conditions such as warmth, moisture and suitable nutrients (6) and enter an inactive state when these are limited. On the contrary, most multicellular systems depend on a steady, adequate supply of oxygen and nutrients essential for their survival and metabolic stability.
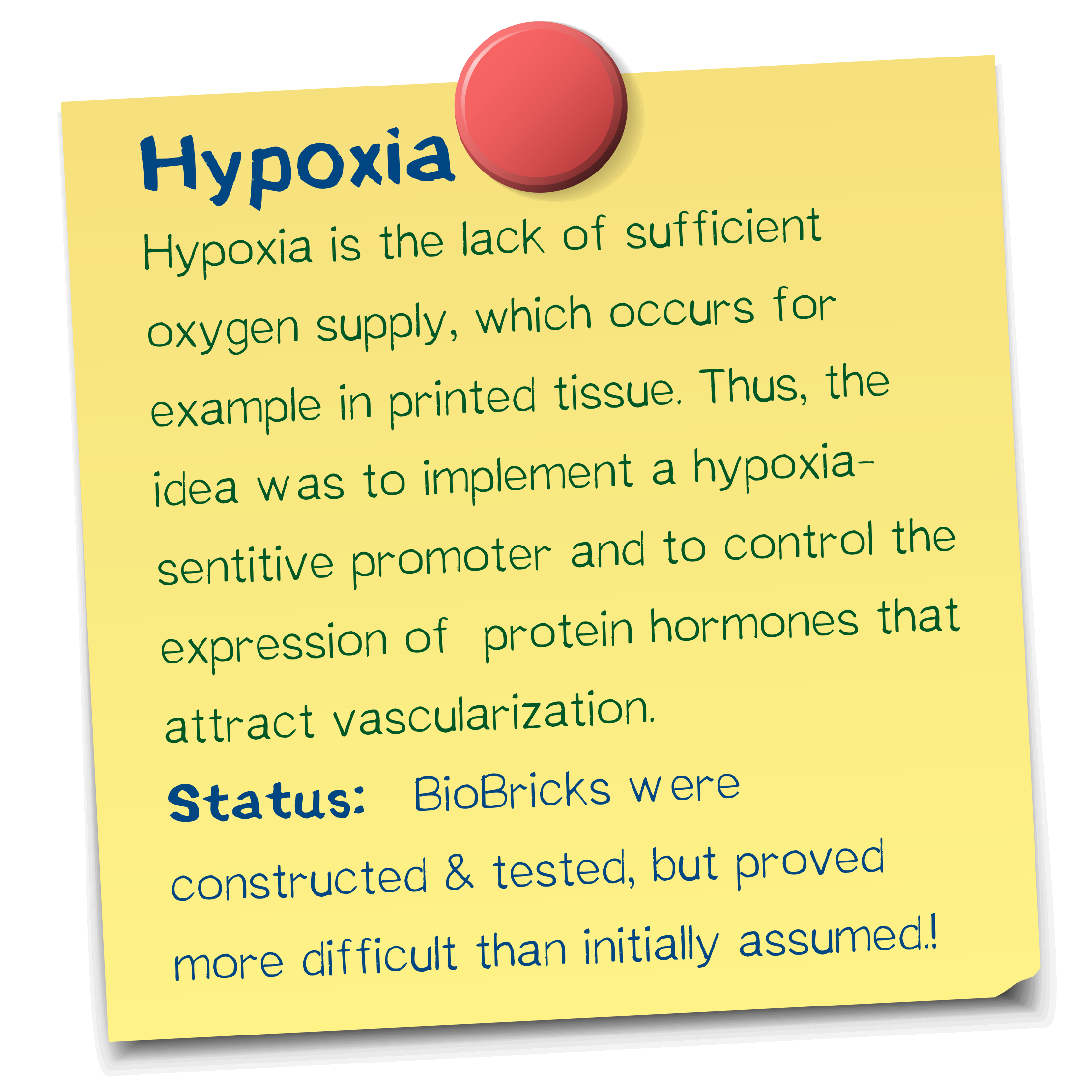
Herein lies one of the main challenges of bioprinting approaches. When printing, the homogenous delivery of oxygen to three-dimensional cell-constructs is limited by diffusion gradients in tissue from the periphery towards the center (7), thus resulting in tissue hypoxia.
In our bodies, this need is met by blood vessels, which form extensive networks that nurture all tissues. As a result, capillaries grow and regress in healthy tissues according to functional demands. (4) Thereby, no metabolically active tissue in the body is more than a few hundred micrometers from a blood capillary, which is formed by the process of angiogenesis. (4)
Sprouting angiogenesis, as implied by its name is characterized by sprouts composed of endothelial cells, which usually grow toward an angiogenic stimulus.
This driving stimulus is initiated in poorly perfused tissues when oxygen sensing mechanisms detect a level of hypoxia that demands the formation of new blood vessels to satisfy the metabolic requirements of parenchymal cells. Most types of parenchymal cells respond to a hypoxic environment by secreting a key proangiogenic growth factor called vascular endothelial growth factor (VEGF-A). (4)
...
Hypoxia-inducible promoters
Constructs and methodology
In order to test hypoxia-dependent gene expression, we created luciferase constructs that allow the quantification of expression levels via a luciferase assay. The expression of these constructs is driven by a hypoxia-inducible promoter, which results in the translation of a luciferase and its secretion into the medium. By taking samples of the medium after 12 h and 24 h after the exposure of HEK293T cells to hypoxia (8% CO2, 92% N2) and measuring luciferase activity by a corresponding assay, we are able to quantify hypoxia-dependent gene expression. In order to determine whether gene expression is really only responsive to hypoxia - but inactive during normal oxygen concentrations - a control at normal oxygen concentrations (21%) was furthermore run.
For the assay, a total of eight constructs were tested, six of which are luciferase constructs for the quantification of expression levels and two of which are VEGF-constructs that are used to confirm the expression of the actual growth factor by our cells. Luciferase constructs all contain the hypoxia promoter, a signal peptide, a coding sequence and a polyadenylation signal, but vary in the number of hypoxia-response elements.
Of the two constructs that do not contain a luciferase, but the actual growth factors (VEGF and PDGF), one is expressed via a hypoxia-promoter (4x HRE) and one via a CMV promoter as a positive control. Expression levels are hereby determined via an ELISA.
Results
Discussion
Two interesting observations were made as a result of the luciferase assays and the ELISA.
1) Although two of the nanoluciferase constructs did not seem to be functional (hypoxia promoter with 2x HRE and 4x HRE), a distinct tendency could be observed for the others - showing that the number of HREs positively correlates with expression levels - the construct with 8x HREs showing the highest absolute secretion of luciferases.
2) Expression levels compared between hypoxia and normoxia do not significantly differ, making the promoter created by us not actually responsive towards states of low oxygen. Higher numbers of heat shock repeats thus seem to increase expression levels in total, but not only in response towards hypoxia.
References