Labjournal
Samples
Transformation of E. coli XL1 blue with Phytochrome B (2–908 N-terminal amino acids) (BBa_K801031, RFC25, pSB1C3)
Investigator: Jeff, Rosario
Aim of the experiment: Transformation of Phytochrome B for protein fusion
Procedure:
- CaCl2-competent E. coli XL1-Blue cells were taken from the stock in –80 °C freezer and gently thawed on ice
- 2 µl of DNA was added to 100 µl of competent cells and gently mixed
- 30 min incubation on ice
- 5 min heat shock at 37 °C
- Added 1 ml LB medium to each tube
- Incubation for 45 min at 37 °C in the 180 rpm cell-culture shaker
- 100 µl of the cell suspension was plated on one chloramphenicol plate
- The rest were centrifuged for 1 min at 13,000 rpm and the supernatant was dicarded
- The pellet was resuspended in 100 µl of LB-medium and this concentrated cell suspension was plated again on a new chlorampenicol plate.
Miniprep of pTUM100 with pGAL, pTEF1, pTEF2, pADH and RFC25 compatible RFP generator
Investigator: Jeff, Rosario
Aim of the experiment: Miniprep of pTUM100 with pGAL, pTEF1, pTEF2, pADH and RFC25 compatible RFP generator
Procedure:
- Miniprep was performed after manufacturer's protocol (QIAprep Miniprep, QIAGEN)
Sequencing of RFP-Generator (RFC25, pSB1C3)
Investigator: Jeff, Rosario
Aim of the experiment: Sequencing of RFP-Generator (RFC25, pSB1C3)
Procedure:
Sequencing batch were prepared after manufacturer's protocol. (15 µl of plasmid DNA (50 - 100 ng) and 2 µl sequencing primer)
Picking of of E. coli XL1 blue with Phytochrome B (2-908 N-terminal amino acids) (BBa_K801031, RFC25, pSB1C3)
Investigator: Jeff, Rosario, Florian
Aim of the experiment: Picking of of E. coli XL1 blue with Phytochrome B (2-908 N-terminal amino acids) (BBa_K801031, RFC25, pSB1C3)
Procedure:
- pSB1C3 plasmid with BBa_K801031 (PhyB 2 - 908 aa, RFC25): Colonies were picked from chloramphenicol plates.
- Picked pipette tips was transferred into cell-culture tubes with air-permeable, sterile cover. Each tube contain 4 mL of LB-medium + 4 µL chloramphenicol(1000x).
- 4 colonies were picked.
- These tubes were transferred in a cell culture shaker at 37 °C and were incubated overnight
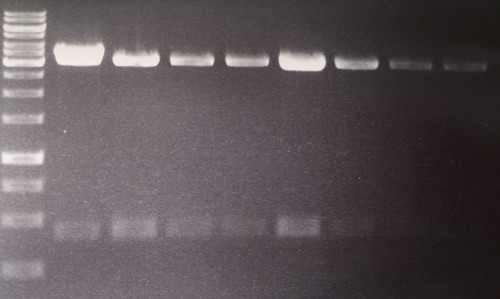
Analytical digestion and gelelectrophoresis of RFP-generator (RFC25, pSB1C3, P4 & P5)
Investigator: Jeff, Rosario, Florian
Aim of the experiment: Analytical digestion and gelelectrophoresis of RFP-generator (RFC25, pSB1C3, P4 & P5).
Procedure:
- Batch for analytical digestion for P4 with NgoMIV+AgeI-HF
| volume | reagent |
| 2.5 µl | Plasmid DNA P4 |
| 2 µl | NEBuffer 4 (10x) |
| 0.25 µl | NgoMIV (10 U/µl) |
| 0.25 µl | AgeI-HF (20 U/µl) |
| 15 µl | ddH2O |
| =20 µl | TOTAL |
- Batch for analytical digestion for P5 with NgoMIV+AgeI-HF
| volume | reagent |
| 2.5 µl | Plasmid DNA P5 |
| 2 µl | NEBuffer 4 (10x) |
| 0.25 µl | NgoMIV (10 U/µl) |
| 0.25 µl | AgeI-HF (20 U/µl) |
| 15 µl | ddH2O |
| =20 µl | TOTAL |
- Incubation for 90 min at 37 °C.
- Analytical gelelectrophoresis was performed at 90 V for 60 min.
Results:
| 1 kbp ladder DNA ladder | P4 | P5 |
| Mutation successful | Mutation successful! |
- Parts are compliant and do not contain RFC25 forbidden restriction sites.
Sequencing of pTUM vectors with pGAL, pADH, pTEF1, pTEF2
Investigator: Jeff, Rosario, Florian
Aim of the experiment: Sequencing of pTUM vectors with pGAL, pADH, pTEF1, pTEF2
Procedure:
Sequencing batch were prepared after manufacturer's protocol. (15 µl of plasmid DNA (50 - 100 ng) and 2 µl sequencing primer).
The different vectors we sequenced received the following barcodes:
- ADH in pTUM100: FR01002265
- TEF1 in pTUM100: FR01002266
- TEF2 in pTUM100: FR01002266
- GAL in pTUM100: FR01002268
Sequencing of TEF2 in pTUM100 was not interpretable. The other sequences were consistent with the sequences in the parts registry.
Miniprep of Phytochrome B (2-908 N-terminal amino acids) (BBa_K801031, RFC25, pSB1C3)
Investigator: Jeff, Florian
Aim of the experiment: Miniprep of Phytochrome B (2-908 N-terminal amino acids) (BBa_K801031, RFC25, pSB1C3).
Procedure:
- Miniprep was performed after manufacturer's protocol (QIAprep Miniprep, QIAGEN)
Analytical digestion and gelelectrophoresis of Phytochrome B (2-908 N-terminal amino acids) (BBa_K801031, RFC25, pSB1C3), P7 - P10
Investigator: Jeff, Florian
Aim of the experiment: Analytical digestion and gelelectrophoresis of Phytochrome B (2-908 N-terminal amino acids) (BBa_K801031, RFC25, pSB1C3), P7 - P10.
Procedure:
- Batch for analytical digestion for P7 with NgoMIV+AgeI-HF
| volume | reagent |
| 2.5 µl | Plasmid DNA P7 |
| 2 µl | NEBuffer 4 (10x) |
| 0.25 µl | NgoMIV (10 U/µl) |
| 0.25 µl | AgeI-HF (20 U/µl) |
| 15 µl | ddH2O |
| =20 µl | TOTAL |
- Batch for analytical digestion for P8 with NgoMIV+AgeI-HF
| volume | reagent |
| 2.5 µl | Plasmid DNA P8 |
| 2 µl | NEBuffer 4 (10x) |
| 0.25 µl | NgoMIV (10 U/µl) |
| 0.25 µl | AgeI-HF (20 U/µl) |
| 15 µl | ddH2O |
| =20 µl | TOTAL |
- Batch for analytical digestion for P9 with NgoMIV+AgeI-HF
| volume | reagent |
| 2.5 µl | Plasmid DNA P9 |
| 2 µl | NEBuffer 4 (10x) |
| 0.25 µl | NgoMIV (10 U/µl) |
| 0.25 µl | AgeI-HF (20 U/µl) |
| 15 µl | ddH2O |
| =20 µl | TOTAL |
- Batch for analytical digestion for P10 with NgoMIV+AgeI-HF
| volume | reagent |
| 2.5 µl | Plasmid DNA P10 |
| 2 µl | NEBuffer 4 (10x) |
| 0.25 µl | NgoMIV (10 U/µl) |
| 0.25 µl | AgeI-HF (20 U/µl) |
| 15 µl | ddH2O |
| =20 µl | TOTAL |
- Incubation for 90 min at 37 °C.
- Analytical gelelectrophoresis was performed at 90 V for 60 min.
Results:
| 1 kbp ladder DNA ladder | P7 | P8 | P9 | P10 |
| Part is correct | Part is correct | Part is correct | Part is correct |
Transformation of E. coli XL1 blue with
Investigator:
Aim of the experiment: Transformation of E. coli XL1 blue.
Procedure:
- CaCl2 competent E. coli XL1-Blue cells were put out from the stock in -80 °C freezer and were gently thawed on ice.
- µl of DNA was added to 100 µl of competent cells and gently mixed.
- 30 min incubation on ice
- 5 min. heat shock at 37 °C
- Adding of µl LB-medium to each tube.
- The cell suspension was plated on ampicillin plates (inclusive rescue plate) and incubated over night at 37 °C in the cell-culture shaker.
Week 1, May 16th - May 22nd
Monday, May 16th
Streptavidin Plasmid Check
Investigator: JB, LK, JH
Aim of the experiment: Verify cloning
Procedure:
- MiniPrep following manufacturer's protocol (QIAprep MiniPrep, Qiagen) (4 clones each of pSA1, pSAm1 in pASK75)
- Analytic digestion with 0.25 µL XbaI, 0.25 µL HindIII (HF), 1 µL SmartCut Buffer, 5 µL plasmid DNA, 3.5 µl H2O
- 5 µL analyzed in gel electrophoresis (1% agarose)
Results: Successful cloning verified. Stored at –20 °C
- Lane 1: 5 µL Thermo Fisher, 1 kb ladder
- Lanes 2–9: 5 µL digestions of P6–P13, band of SA (mut1) at about 300 bp, band of digested plasmid at about 3,000 bp
Streptavidin Expression: Transform BL21
Investigator: JB, JH
Aim of the experiment: Express pSA1 and pSAm1 in E. coli BL21
Procedure:
- Transformation into competent E. coli BL21 according to protocol of P6 and P10
Results: Plates (LB Amp) in incubator (37 °C) for further processing
Tuesday, May 17th
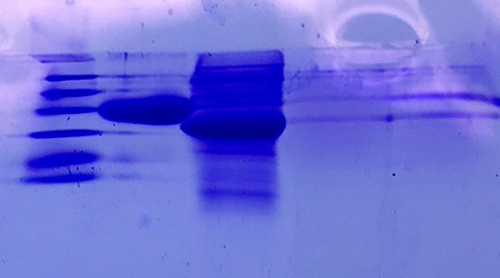
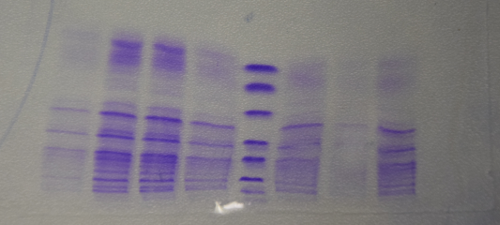
SDS Gel Analysis
Investigator: CG
Aim of the experiment: Analyze collagen 1/2, eGFP, fraction 30 of egg precipitation via SDS gel
Procedure:
- Mixed 80 µL samples with 20 µL SDS buffer
- Heated at 95 °C for 10 min
- 1 d staining, 1 d unstaining
Results: Successful cloning verified. Stored at –20 °C
- Lane 1: 8 µL marker (Thermo Fisher #26610)
- Lane 2: Fraction 30 (IEC), 3 µL, band at 35 kDa, avidin expected at 16 kDa
- Lane 3: eGFP, 12 µL, band at 27 kDa, eGFP expected at 27 kDa, many impurities
- Lane 4: Collagen 1, 12 µL, no sharp band
- Lane 5: Collagen 1, 12 µL, no sharp band
MiniPreps pSb1C3–AviTag, –A3C5 and pASK75–(SA1), –(SAm1)
Investigator: CR, CG
Aim of the experiment: Verify cloning
Procedure:
- MiniPrep was performed following manufacturer's protocol (QIAprep MiniPrep, Qiagen)
- Analytic digestion with 0.25 µL XbaI, 0.25 µL HindIII (HF) for pASK plasmids and 0.25 µL NgomIV, 0.25 µL AgeI (HF) for pSb1C3 plasmids, 1 µL SmartCut Buffer, 5 µL plasmid DNA, 3.5 µL H2O
- 5 µL on 1% agarose gel electrophoresis
Results: Successful cloning verified for pASK plasmids. Repeat pSb1C3 plasmids. Stored at –20 °C
- Lane 1: 5 µL Thermo Fisher, 1 kb Ladder
- Lane 2: 5 µL digestion of pSb1C3–AviTag
- Lane 3: 5 µL digestion of pSb1C3–A3C5
- Lane 5: 5 µL digestion of pASK75(SA1), EB elution
- Lane 6: 5 µL digestion of pASK75(SAmut1), EB elution
- Lane 7: 5 µL digestion of pASK75(SAmut1), H2O elution
Inoculation of Pre-Culture with BL21 (pASK75 (SA1)) in LB Medium
Investigator: CR
Aim of the experiment: Pre-culture for streptavidin expression in TB medium
Procedure:
- Added 50 µL ampicillin to 50 mL LB medium
- Picked colonies from BL21 (pASK75 (SA1))
- Inoculated LB medium
- Incubated at 30 °C overnight
Wednesday, May 18th
Repeat Analytical Gel of pSb1C3–AviTag, –A3C5
Investigator: CG, CR
Aim of the experiment: Verify cloning
Procedure:
- Analytic digestion with 0.25 µL NgoMIV, 0.25 µL AgeI (HF) for pSb1C3 plasmids, 1 µL SmartCut Buffer, 8.5 µL plasmid DNA
- 10 µL analyzed in gel electrophoresis (2% agarose)
Results:
Inoculation of BL21 (pASK75 (SA1)) Culture in 2 L TB Medium and Induction of Streptavidin Production by Addition of Tetracycline
Investigator: CR
Aim of the experiment: Produce streptavidin
Procedure:
- Ampicillin (2 mL) was added to the medium (1:1000)
- The pre-culture (50 mL) was poured into the medium
- Culture incubated at 37 °C and 140 rpm until OD550 reached 0.5
- To induce streptavidin expression, anhydro-tetracycline (200 µL) was added to the culture (1:10,000)
- The culture was incubated at 37 °C and 140 rpm for 4 hours
Results: Streptavidin expression by BL21
Expression and Harvest of Streptavidin (pASK75 (SA1)) in BL21 in TB Medium
Investigator: CR, JB, JH
Aim of the experiment: Recombinant expression and purification of streptavidin
Procedure:
- After expression, cultures were transferred into centrifuge tubes and spun down in the centrifuge (4 °C, 5000 rpm, 20 min, F4X1L rotor)
- The supernatant was discarded, and the pellet was transferred into a beaker of sufficient size and resuspended in Tris Buffer B cooled to 4 °C (50 mM Tris/HCl pH = 8.0, 1 mM EDTA)
- The solution was homogenized in the PANDA (ask supervisor)
- The resulting lysate was transferred into centrifuge tubes and spun down (4 °C, 18,000 rpm, 10 min, XX34-rotor). The supernatant was discarded, and the pellet was resuspended in 6 M Gua-HCl (pH = 1.5) at 4 °C overnight.
Dialysis of eGFP
Investigator: NA, JH, CR
Aim of the experiment: Purify eGFP
Procedure:
- eGFP was thawed on ice
- eGFP was then poured into a dialysis hose (cut-off 14 kDa)
- The hose was then placed in ice-cold Tris/HCl 20 mM pH 8.0
- Dialyzed at 4 °C overnight (XX34 rotor). The supernatant was discarded, and the pellet was
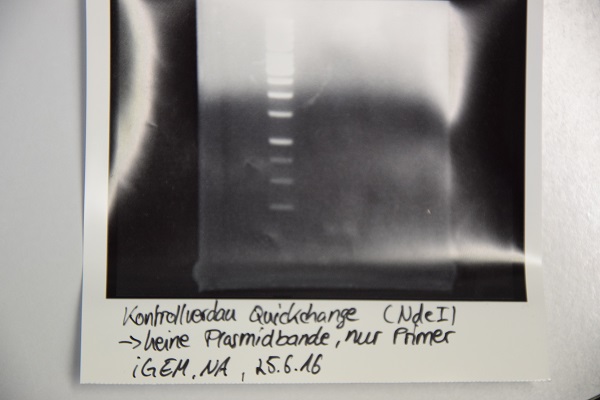
MiniPrep of quickchanged pNGAL146-A2 EspP
Investigator: NA
Aim of the experiment: Extraction of pNGAL146-A2 plasmid from XL1 blue
Procedure:
- MiniPrep was performed after manufacturer's protocol (QIAprep MiniPrep, Qiagen)
Sequencing of P14 (Avi-Tag), P15 (A3C5) & P19 (Quick change (QC) EspP in pNGAL-A2)
Investigator: CR, NA
Aim of the experiment: Sequencing of P14 (Avi-Tag), P15 (A3C5) & P19 (Quick change (QC) EspP in pNGAL-A2)
Procedure:
- Sequencing batches were prepared after manufacturer's protocol (15 µL plasmid DNA (50-100 µM) and 2 µL sequencing primer)
- The different plasmids we prepared received the following barcodes:
- P14 : FR11326653
- P15 : FR11326655
- P19 (K4): FR11326654
- P16 (K1): FR11326652
- P17 (K2): FR11326651
- P18 (K3): FR11326650
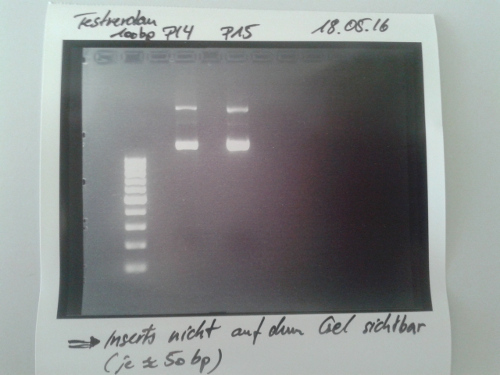
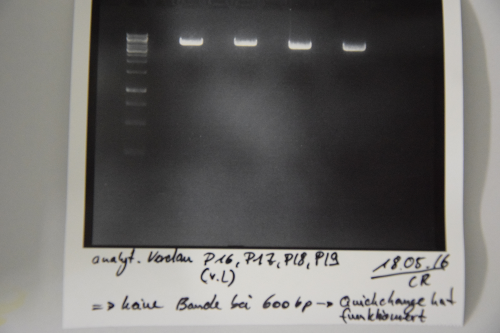
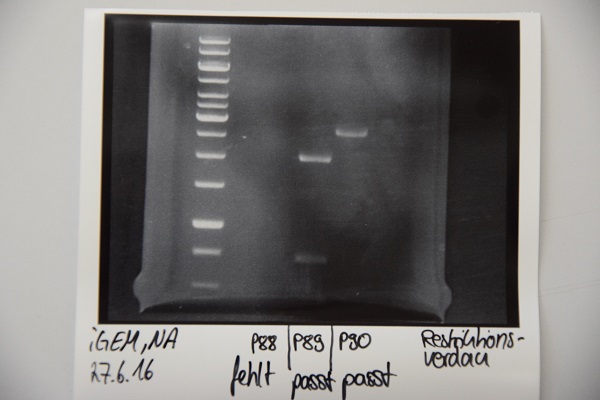
Digestion of P16 , P17, P18 & P19 (all QC EspP in pNGAL-A2) with AgeI & HindIII + analytical gel
Investigator: NA, JH, CR
Aim of the experiment: Verification of success of quickchange
Procedure:
- analytic digestion with: 0,25 µl HindIII (HF), 0,25 µl AgeI (HF), 1 µl SmartCut Buffer, 500 ng plasmid-DNA, fill up with ddH2O (Vtotal= 10µL)
- 10 µl on 2% agarose gel for electrophoresis
Results: No signal at 600 bp --> quickchange seems to be successful (waiting for sequencing)
Thursday, May 19th
Re-Sequencing of P19
Investigator: CR
Aim of the experiment: Re-Sequencing of P19
Procedure:Sequencing batches were prepared after manufacturer's protocol (15 µL plasmid DNA (50-100 µM) and 2 µL sequencing primer)
The different plasmids we prepared received the following barcodes:
- P19 (K4): FR11326649
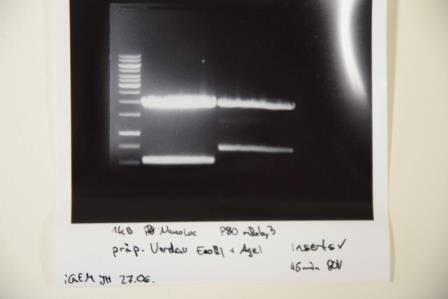
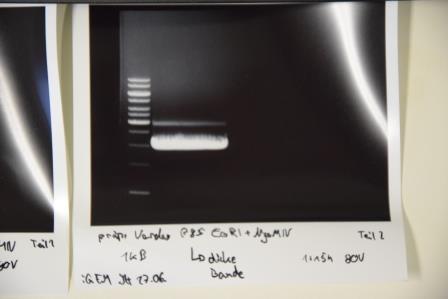
Cloning of A3C5 and Avi-Tag into pSB1C3
Investigator: CG
Aim of the experiment: Re-Trafo of pSB1C3 RFP for later on: digestion, dephosphorylation and cloning
Procedure: transformation according to protocol of P4 E. Coli XL1
Result: plates (LB Cam) in incubator for further processing (37 °C)
Streptavidin refolding
Investigator: JB
Aim of the experiment: Refolding of denaturated Streptavidin
Procedure: After the pellet had almost completely dissolved in 6M GdmCl, the solution was spun down (4°C, 20 mins, 18,000 rpm). The supernatant was transferred carefully into a falcon tube and the pellet was cast away. Via a hydraulic pump (flow rate: 2x10 ml/min) the lysate was transferred Into 5L PBS 1x. Afterwards the pump was cleaned with technical isopropanol and ELGA water. The solution was stirred overnight at 4°C for refolding.
Biotinylation of BSA
Investigator: JB
Aim of the experiment: Biotinylation of BSA
Procedure: A 100 µM (=6.8 mg/ml) solution of BSA (Albumin fraction V, pH=7, in the fridge in the central lab) was created (V=10 ml). 220 µl of a 100 mM Biotin stock were added. The mixture was stored overnight Iin the fridge (4°C).
Result: Hopefully biotinylated BSA mixture in the fridge (4°C).
Friday, May 20th
Qualitative analysis of streptavidin expression
Investigator: CG
Aim of the experiment: SDS gel analysis of recombinant strepatividin expression
Procedure: mixing of 80 µl sample with 20 µl SDS buffer and heating at 95°C for 10 min. 1 h staining, 1 night unstaining
Result:
- 1. Lane: 8 µl Marker (Thermo Fisher #26610)
- 2. Lane: 12 µl culture aliquot, before induction
- 3. Lane: 12 µl culture aliquot, after induction
- 4. Lane: 3 µl culture aliquot of the lysed pelet, Strepatvidin expected at about 16 kDa
- 5. Lane: 3 µl culture aliquot of the supernatend after lysis, no Strepatvidin expected at about 16 kDa
- 6. Lane: 1.5 µl culture aliquot of the lysed pelet, Strepatvidin expected at about 16 kDa
- 7. Lane: 1.5 µl culture aliquot of the supernatend after lysis, no Streptavidin expected at about 16 kDa
Cloning of A3C5 and Avi-Tag into pSB1C3
Investigator: LK
Aim of the experiment: Inoculation of pre-culture with E. coli XL1 (pSb1C3 -RFP) in LB-Chloramphenicol-medium
Procedure: Picking of colonies for E. coli XL1 (pSb1C3 -RFP) Inoculate in 5 ml LB-Chloramphenicol-medium Incubate at 37°C over night at 200 rpm
Transformation of Biobricks in XL1-blue
Investigator: NA, JH
Aim of the experiment: Transformation
Procedure: -10 µl dd H2O in well of interest (standard distribution kit) -1 µl Plasmid (out of well) to cells -Transformation according to the SOP Used bricks: K577893, B0015, R0040, B0032, I14033, K747096
Ammonium sulfate precipitation of streptavidin
Investigator: JB
Aim of the experiment: Reduction of the protein solution volume and precipitation of streptavidin
Procedure: The 5 L protein solution was spun down (20 mins, 5,000 rpm) and the supernatant transferred into a beaker. In order to lower the volume of the solution for ammonium sulfate precipitation, the solution was first filtered via a membrane crossflow pump (membrane: Sartocon 0.45 µm, thick membrane).
Dialysis of biotinylated BSA
Investigator: NA
Aim of the experiment: purification of biotinylated BSA
Procedure: dialysis against Tris/HCl 20 mM, pH 8, 10 mM NaCl over night; cut off : 14 kDa
Week 2 (May 23rd - May 29th)
Monday, May 23th
Cloning of A3C5 and Avi-Tag into pSB1C3
Investigator: CR
Aim of the experiment: Cloning of A3C5 and Avi-Tag into pSB1C3
Procedure:
- MiniPrep was performed after manufacturer's protocol (QIAprep MiniPrep, Qiagen)
- pSB1C3 was digested with AgeI and NgOMIV (10 µL plasmid + 1.5 µL AgeI + 1.5 µL NgoMIV + 3 µL CutSmart + 14 µL ddH2O; incubation for 1h, 37°C)
- FastAP was added and the reaction was incubated for another 2h at 37°C
- The digestion was purified by gelelctrophoresis (1%, 75V, 1h)
- Plasmid backbone was cut from the gel
Results:
Signal 1: linearized pSB1C3 (successfull digestion)
Signal 2: supercoiled pSB1C3 (digestion not successfull)
Signal 3: RFP-generator
--> next time gel should run longer (just if you need the back bone)
Filtration of Streptavidin and precipitation with Ammonium-sulfate
Investigator: MP, CR
Aim of the experiment: Concentration of Streptavidin
Procedure: The solution was filtered via a membrane crossflow pump (membrane: Sartocon 0.45 µm, thick membrane). The final volum was 0.5L The solution was precipitated by addition of Ammonium-sulfate (2 steps: 1. 40% Ammonium-sulfate; 2. 70% Ammonium-sulfate) After the first addition of ammonium-sulfate the solution was stirred (1h, 4°C) Afterwards the solution was centrifuged (10.000 rpm; 30 min) and the supernatant was used for the second precipitation step The solution was stirred again (over night, 4°C)
Inoculation of pre-culture with BioBricks in pSB1C3 in LB-Cam
Investigator: JH, JL
Aim of the experiment:
Procedure: Add 50 µL chloramphenicol in 50 mL LB-medium Picking colonies from K577893; B0015; B0032 (in pSB1C3) * Inoculate 4 mL medium (CAM) Incubate at 37°C over night
- Following cultures didn´t grow and were therefore not inoculated:
R0040; K747096; I14033
Retransformation of Biobricks in XL1-blue
Investigator: JH, JL, EF
Aim of the experiment: Transformation
Procedure: 10 µl dd H2O in well of interest (standard distribution kit) 1 µl Plasmid (out of well) to cells Transformation according to the SOP Used bricks: K577893, B0015, R0040, B0032, I14033, K747096
Tuesday, May 24th
Inoculation of pre-culture with BioBricks in pSB1C3 in LB-Cam
Investigator: CR
Aim of the experiment:
Procedure: Add 50 µL chloramphenicol in 50 mL LB-medium Picking colonies from K577893; B0015; B0032 (in pSB1C3) * Inoculate 4 mL medium (CAM) Incubate at 37°C over night
Cloning of A3C5 and Avi-Tag into pSB1C3
Investigator: CR
Aim of the experiment: Gelextraction of Signal 1 from pSB1C3-digestion
Procedure: Gelextraction was performed after manufacturer's protocol (Gelextractionkit, Qiagen)
Ammonium sulfate precipitation
Investigator: JB
Aim of the experiment: Precipitation the 70% saturated ammonium sulfate solution
Procedure: The ammonium sulfate solution, which actually was a suspension, was transferred into centrifuge tubes and spun down (10,000 rpm, 45 mins). As it turns out, unfortunately too much ammonium sulfate was used for the precipitation (wrong table). The precipitate was brought back into solution (20 mM Tris/HCl, pH 8.0) and loaded onto an SDS gel for analysis, along with other samples (flowthrough of the filtration from the day before).
MiniPrep of BioBricks in pSB1C3 (inoculated on May 23th)
Investigator: JH
Aim of the experiment: Extraction of BioBricks B0032, B0015 and K577893 in pSB1C3 from XL1 blue
Procedure: MiniPrep was performed after manufacturer's protocol (QIAprep MiniPrep, Qiagen) concentrations measured (in ng/µL): (B0032) 360.2; (B0015) 254.9; (K577893) 99.2
Sequencing of P23 (weak RBS), P24 (double terminator) & P24 (Tet repressed GFP)
Investigator: JH
Aim of the experiment: Sequencing of P23, P24 & P25 (x2)
Procedure: Sequencing batches were prepared after manufacturer's protocol (15 µL plasmid DNA (50-100 µM) and 2 µL sequencing primer)
The different plasmids we prepared received the following barcodes:
- P23 (VF2) : FR11326647
- P24 (VF2) : FR11326646
- P25 (VF2) : FR11326645
- P25 (VR) : FR11326644
Wednesday, May 25th
Inoculation of pre-culture with tet-repressed GFP (P25) in LB-Cam
Investigator: NA
Procedure: Add 50 µL chloramphenicol to 50 mL LB-medium Picking colonies from plate (BL21) Incubate over night at 37°C => dismissed, because plasmid sequence was inaccurate
MiniPrep of K747096, I14033, R0040, B0015, K577893 and B0032
Investigator: CR
Aim of the experiment: MiniPrep of BioBricks
Procedure: MiniPrep was performed after manufacturer's protocol (QIAprep MiniPrep, Qiagen) Concentrations were measured (in ng/µL):
- K747096: 286.2
- I14033: 246.1
- R0040: 243.5
- B0015: 244.1
- K577893: 440.9
- B0032: 259.7
PCR of P19 (QC EspP) with O3 and O4
Investigator: CR
Aim of the experiment: Amplification of quickchanged EspP
Procedure:
- reaction was performed after manufacturer's protocol (PCR Using Q5® High-Fidelity DNA Polymerase, NEB)
- 274,4 pg Plasmid were used for amplification (1 µL)
- PCR program (standard):
| Step | Temperature [°C] | Time [s] |
| initial Denaturation | 98 | 30 |
| 35 cycles | 98 | 10 |
| 72 | 30 | |
| 72 | 60 | |
| final Extension | 72 | 5 |
| Hold | 4 | ꝏ |
- PCR product was purified accoding to manufacturer's protocol (PCR puification kit, Qiagen)
- DNA was eluted 40 µL EB buffer
Digestion of PCR of EspP (P19 with O3 and O4)
Investigator: CR
Aim of the experiment: Digestion of EspP with AgeI and NgoMIV to ligate it in pSB1C3 later on
Procedure:
- Batch for preparative digestion:
| Volume | Reagent |
| 38.5 µL | PCR product P19 with O3 and O4 |
| 1 µL | AgeI |
| 1 µL | NgoMIV |
| 5 µL | CutSmart buffer |
| 4.5 µL | ddH2O |
| 50 µL | TOTAL |
- Incubation for 2 h at 37 °C
Sequencing of P26 (CMV promotor), P27 (p cat promotor), P28 (Tet repressible promotor), P30 (Tet repressed GFP)
Investigator: NA
Aim of the experiment: Sequencing of P26, P27, P28 & P30 (x2)
Procedure: Sequencing batches were prepared after manufacturer's protocol (15 μL plasmid DNA (50-100 μM) and 2 μL sequencing primer) The different plasmids we prepared received the following barcodes: P26 (VF2) : FR11326643 P27 (VF2) : FR11326642 P28 (VF2) : FR11326641 P30 (VF2) : FR11326640 P30 (VR): FR11326639
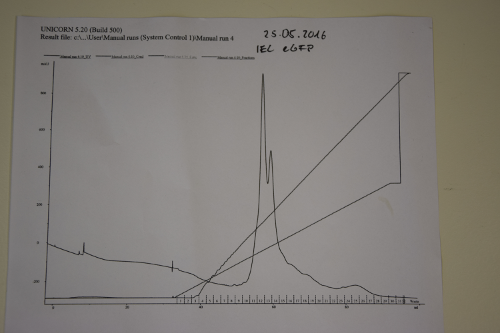
Ion exchange chromatography of eGFP
Investigator: NA, JB,JL
Aim of the experiment: purification of eGFP
Procedure:
- use Äkta-Purifier (Q-column for eGFP (quarternary ammonium as stationary phase)). Ask Andy before use!
- Pump A: running buffer (20 mM Tris/HCl pH 8); pump B: elution buffer (20 mM Tris/HCl pH 8 and 1,000 mM (1 M) NaCl
- basic pumpwash before start
- injection in loop
- gradient of ion strength on column.
Results: Elution of eGFP at ~200 mM NaCl.
Thursday, May 26th
Inoculation of cultures with XL-1 F11 and F12 clones
Investigator: JB
Aim of the experiment: Inoculation of a 5 ml culture with a clone of the previously transformed F11 (Avi-Tag) F12 (Antibody-binding site).
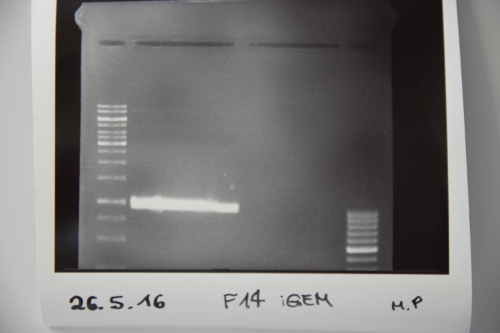
Cloning of ligation F14 +F13
Investigator: MP, EF, JH
Aim of the experiment: new biobrick EspP in pSB1C3
Procedure: F14 was purified by gelelctrophoresis (1%, 90 V, 40 min) gelextraction was performed after manufacturer's protocol (Gelextractionkit, Qiagen) ligation of F14 (EspP) with F13 (dephos. PSB1C3) transformation according to protocol in competent E.coli XL1 blue
Results:
Transformation of Biobrick B0034 in XL1-blue
Investigator: JH
Aim of the experiment: Transformation
Procedure:
- 10 µl ddH2O in well of interest (standard distribution kit, plate 4, well 1N)
- 1 µl Plasmid (out of well) to cells
- Transformation according to the SOP (incl. rescue)
Transformation of Biobricks K577895 and K577894 in XL1-blue
Investigator: NA
Aim of the experiment: Transformation
Procedure:
- 10 µl ddH2O in well of interest (standard distribution kit, plate 1, wells 14B / 12P)
- 1 µl Plasmid (out of well) to cells
- Transformation according to the SOP (incl. rescue)
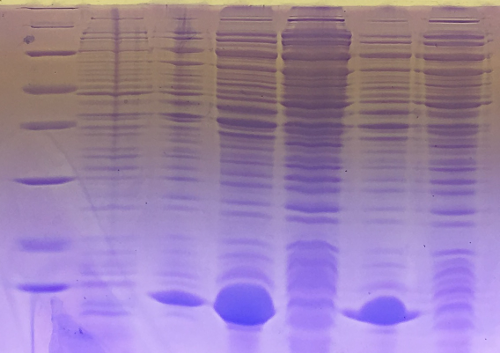
SDS-PAGE of eGFP-fractions after IEC
Investigator: NA, CG
Procedure: mixing of 80 µl sample with 20 µl SDS buffer and heating at 95°C for 10 min. 1 h staining, 1 night unstaining Concentrations were measured via nanodrop (A280, extinction coefficient: 55000 M−1cm−1)
Result: concentrations in mg/ml 1. Lane: 8 µl Marker 2. Lane: 10 µl of fraction 10; c= 0 3. Lane: 10 µl of fraction 11; c= 0,15 4. Lane: 10 µl of fraction 12; c= 0,77 5. Lane: 10 µl of fraction 13; c= 0,29 6. Lane: 10 µl of fraction 14; c= 0,4 7. Lane: 10 µl of fraction 15; c= 0,144 8. Lane: 10 µl of fraction 16; c= 0,085
eGFP is clearly detectable at about 27 kDa. The most pure fractions 12 and 13 were pooled for later biotinylation.
Friday, May 27th
Inoculation of pre-cultures with B0034 (strong RBS), K577894 (Tet repressed YFP), KK577895 (Tet repressed RFP), F13+F14
Investigator: JH
Procedure:
- Add 50 µL ampicillin(B0034)/chloramphenicol(K5777894, K577895, F13+F14) to 50 mL LB-medium
- Picking colonies from plate (XL1 blue; all rescue)
- Incubate over night at 37°C
Inoculation of BL21 (pASK75 (SA1)) culture in 2 L TB-Medium and induction of streptavidin production by addition of tetracycline
Investigator: NA
Aim of the experiment: Production of streptavidin
Procedure:
- Ampicillin (2 mL) was added to the Medium (1:1000)
- The pre-culture (50 mL) was poured into the Medium
- Culture was incubated at 37°C and 140 rpm until OD550 reached 0.5
- To induce streptavidin expression anhydro-tetrazycline (200 µL) was added to the culture (1:10000)
- The culture was incubated at 37°C and 140 rpm for 4 hours
Result: Streptavidin expression by BL21
Re-sequencing of P30 (Tet repressed GFP in pSB1C3)
Investigator: NA
Aim of the experiment: Purification and sequencing of P30
Procedure:
- P30 was heated up to 100 °C and centrifugated afterwards to remove proteins from Promotor (if there are some), the supernatant is sequenced
- Sequencing batches were prepared after manufacturer's protocol
- (15 μL plasmid DNA (50-100 μM) and 2 μL sequencing primer)
- The different plasmids we prepared received the following barcodes:
- P30 (VF2) : FR11326638
Expression and harvest of Streptavidin (pASK75 (SA1)) in BL21 in TB-medium
Investigator: JH, NA
Aim of the experiment: Recombinant expression and purification of Streptavidin
Procedure:
- After expression, cultures were transferred into centrifuge tubes and spun down in the centrifuge (4°C, 5000 rpm, 20 mins, F 4X1L rotor)
- The supernatant was cast away and the pellet was stored at –20 °C
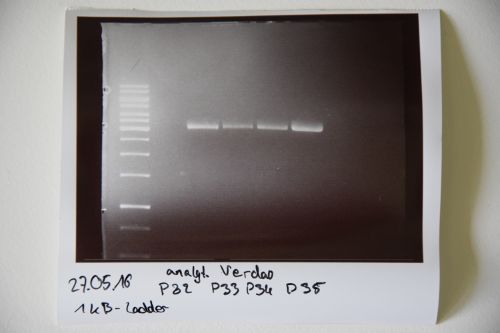
Cloning of A3C5 and Avi-Tag into pSB1C3
Investigator: CG
Aim of the experiment: MiniPrep of cloned pSB1C3-A3C5 and –AviTag Plasmids
Procedure:
- MiniPrep of inoculated precultures was performed according to manufacturer’s protocol (QIAprep MiniPrep, Qiagen)
- Concentrations were measured (in ng/µL):
Colony 1 of pSB1C3- AviTag P32: 120 Colony 2 of pSB1C3- AviTag P33: 110 Colony 1 of pSB1C3-A3C5 P34: 126 Colony 2 of pSB1C3-A3C5 P35: 188
Cloning of A3C5 and Avi-Tag into pSB1C3
Investigator: NA, JH, CG
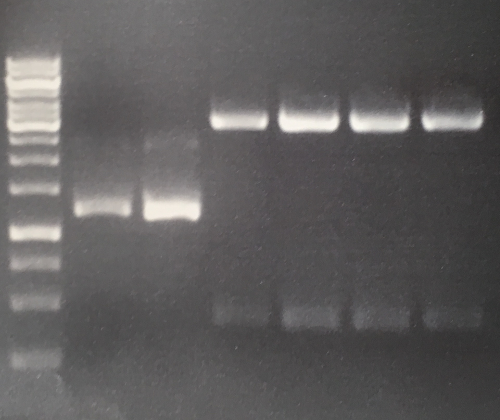
Aim of the experiment: Initial vector digestion was performed with NgoMiV and AgeI, producing complementary overhangs. Religated pSB1C3 (due to incomplete vector dephosphorylation) loses the AgeI recognition sequence. Therefore plasmids without insert should run in the supercoiled position at about 1.5 kb, plasmids with inserts should run in the linearised position at 3 kb. Single enzyme digestion was chosen, because the insert is too small to be solved on a gel.
Procedure:
- analytic digestion: 3 µl of plasmid DNA P32 to P35 (about 300 ng) were digested with 0.3 µl AgeI-HF in 1 µl CutSmart Buffer and 5.7 µl H2O. The reaction was incubated for 2 h at 37°C.
- 5 µl digestion mix were loaded on a 1% agarose gel for electrophoresis, 1 kb ladder
Result:
- P32 to P35 might carry the insert because they all appear in the linearized position at 3 kb
- Therefore plasmid sequencing should confirm the result.
Lane 1: 1 kb ladder Lane 3: P32 Lane 4: P33 Lane 5: P34 Lane 6: P35
Week 3 (May 30th - June 5th)
Monday, May 30th
Cloning of A3C5 and Avi-Tag into pSB1C3
Investigator: CR
Aim of the experiment: Sequencing of P32 and P35
Procedure:
- Sequencing batches were prepared according to manufacturers protocol (Mix2Seq Kit, Eurofins Genomics): 15 μL plasmid DNA (50-100 μM) and 2 μL sequencing primer (10 µM)
- The plasmids received the following barcodes:
- P32 (VF2) : FR11326637
- P35 (VF2) : FR11326636
Religation of F13 (digest of pSB1C3 with AgeI and NgoMIV) and transformation in XL1 blue
Investigator: CR
Aim of the experiment: Re-ligate digested and dephosphorylated pSB1C3 and transformation in XL1blue
Procedure:
- Plasmid was ligated with the T4 DNA Ligase following the manufacturer’s protocol (NEB)
- 52 ng Vector DNA were used
- The Ligation mix was incubated for 30 min at RT
- 7 μL Ligation mixed were used for the transformation of 50 μL XL1 blue.
- Transformation was performed following the SOP
Result:
- 5 clones on plate
Inoculation of pre-cultures with B0034 (strong RBS), K577894 (TET repressed YFP), KK577895 (Tet repressed RFP), F13+F14
Investigator: CR
Procedure:
- Add 50 µL ampicillin(B0034)/chloramphenicol(K5777894, K577895, F13+F14) to 50 mL LB-medium
- Picking colonies from plate (XL1 blue; all rescue)
- Incubate over night at 37°C
Tuesday, May 31th
Dialysis of eGFP
Investigator: NA
Aim of the experiment: Changing the buffer of eGFP for biotinylation (from TRIS to PBS)
Procedure:
- eGFP was poured in a dialysis hose (cut-off 14 kDa)
- The hose was then placed in ice cold PBS pH 7.4
- The dialysis took place at 4°C overnight
Expression and harvest of Streptavidin (pASK75 (SA1)) in BL21 in TB-medium
Investigator: JB, NA
Aim of the experiment: Recombinant expression and purification of Streptavidin
Procedure:
- The pellet was transferred into a beaker of sufficient size and resuspended in fridge-cooled Tris Buffer B (50 mM Tris/HCl (pH = 8.0), 1 mM EDTA)
- The solution was homogenized in the PANDA (see SOP)
- The resulting lysate was transferred into centrifuge tubes and spun down (4°C, 18,000 rpm, 30 mins, SS34-rotor). The supernatant was cast away and the pellet was resuspended in 6M Gua-HCl (pH = 1.5) at 4°C overnight.
Transformation of P30 (Tet repressed GFP)
Investigator: NA
Aim of the experiment: expression of tet-repressed GFP in E. Coli BL21
Procedure: transformation in competent E. Coli BL21 according to protocol
Result: plates (LB Cam) in incubator for further processing (37 °C)
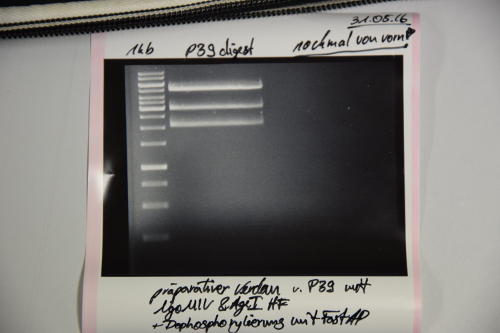
Cloning of A3C5 and Avi-Tag into pSB1C3
Investigator: CR, JH
Aim of the experiment: Preculture of pSB1C3 (F13+F14) was red See if RFP-generator is still inside the pSB1C3 See if ligation of EspP was successful
Procedure:
- MiniPrep of P39 was performed after manufacturer's protocol (QIAprep MiniPrep, Qiagen)
- pSB1C3 was digested with AgeI and NgOMIV (1.5 µL P39 + 1.5 µL AgeI + 1.5 µL NgoMIV + 3 µL CutSmart + 22.5 µL ddH2O; incubation for 2h, 37°C)
- FastAP was added and the reaction was incubated for another 10min at 37°C
- FastAP was inactivated by heating the mix to 75°C for 10min.
- The digestion was purified by gelelctrophoresis (1%, 75V, 1h)
Results:
Unknown signals. Repeat transformation, digestion, dephosphorylation and ligation of pSB1C3
Re-transformation of P4 (pSB1C3 (RFP-generator)) in XL1 blue
Investigator: JH, CR
Aim of the experiment: Transformation
Procedure:
- 2 μL P4 were used for transformation of XL1 blue
- Transformation was performed according to SOP (incl. rescue plate)
Competent E. coli XL1 cells
Investigator: JB
Aim of the experiment: Generation of competent E.coli XL1 cells
Procedure:
- 5 ml LB w.o. antibiotics (sterile)
- Inoculation of one colony of E.coli XL1 cells
- Incubate overnight at 37°C
Wednesday, June 1th
eGFP Biotinylation
Investigator: CG
Aim of the experiment: Biotinylation of dialysed eGFP past IEC
Procedure:
- The average concentration of the pooled factions 12 and 13 should be about 0.5 mg/ml (=19 µM)
- 10 ml of a 100 mM Biotin stock-solution was prepared (340 mg)
- 3.5 ml of eGFP were biotinylated with 12 µl 100 µM Biotin-solution (20x molar excess)
- Incubation at 4 °C overnight
pSB1C3 vector backbone preperation
Investigator: CG
Aim of the experiment: Inoculation of two colonies pSB1C3-RFP Generator
Procedure:
- 2x 5 ml LB+Cam medium (1:1000)
- Picking colonies from plate (XL1 blue, rescue)
- Incubate overnight at 37°C
Generation of GFP expressing E. coli cells
Investigator: CG
Aim of the experiment: Inoculation of BL21 transformed cell with P30
Procedure:
- 50 ml LB+Cam medium (1:1000)
- Picking colonies from plate (BL21, rescue)
- Incubate over night at 37°C
Competent E. coli XL1 cells
Investigator: JB
Aim of the experiment: Generation of competent E. coli XL1 cells
Procedure:
- Inoculated 500 µl preculture in 50 ml LB media w.o. antibiotics
- Incubated and shaken at 37°C until the OD550 reached 0.44
- Transferred into a falcon tube and incubated on ice for 10 mins
- Spun down at 3000 g for 10 mins at 4°C, supernatant cast away
- Pellet resuspended in 40 ml fridge-cooled MgCl2 (100 mM), once again spun down at 4°C for 10 mins at 3000 g
- Supernatant cast away, pellet resuspended in 20 ml cold CaCl2 (50 mM)
- Incubation on ice for 30 mins
- Centrifugation repeated, supernatant cast away
- Pellet resuspended in 2 ml of CaCl2 (50 mM) and glycerol (15%)-solution, aliquoted (50 µl each), frozen in liquid nitrogen and stored at -80°C for further use
Cloning of Pcat-RBS-construct in pSB1A2
Investigator: CG, JH, CR
Aim of the experiment: Pcat (I14033) was cut out of P27 and ligated into P38 for generating the Pcat-RBS (B0034) construct.
Procedure:
- P27 was digested with EcoRI and SpeI (4 µL P27 + 1.5 µL EcoRI + 1.5 µL SpeI + 3 µL CutSmart + 20 µL ddH2O; incubation for 2h, 37°C)
- P38 was digested with EcoRI and XbaI (10 µL P27 + 1.5 µL EcoRI + 1.5 µL XbaI + 3 µL CutSmart + 14 µL ddH2O; incubation for 2h, 37°C)
- Preperative gel electrophoresis was performed
- For P27 the signal at about 50 bp was cut out and gel extracted according to manufacturer's protocol (Gelextractionkit, Qiagen): 5.9 ng/µl
- For P30 the signal at about 2.1 kb was cut out and gel extracted according to manufacturer's protocol (Gelextractionkit, Qiagen): 8.9 ng/µl
- Ligation according to SOP
Purification of streptavidin
Investigator: JB
Aim of the experiment: Refolding denaturized streptavidin
Procedure:
- the GdmCl-solved streptavidin was spun down using an SS-34 rotor (~60 min, 18,000 rpm, 4°C)
- The supernatant containing the solved and denaturized protein was transferred into a falcon tube, the pellet cast away
- The protein solution was slowly dripped into 4 l of cooled 1x PBS (in the 4°C room) using a hydraulic pump and stirred overnight
Tuesday, June 2th
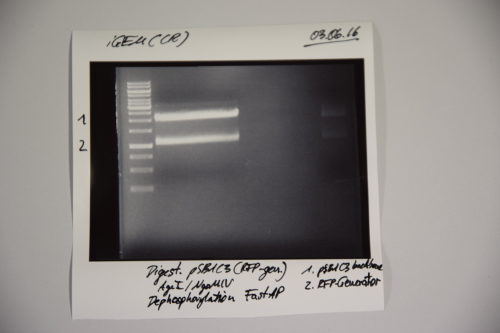
Preparation of pSB1C3 for further use
Investigator: CR
Aim of the experiment: Digestion of pSB1C3 to remove RFP-generator
Procedure:
- MiniPrep was performed after manufacturer's protocol (QIAprep MiniPrep, Qiagen)
- P40 was digested with AgeI and NgOMIV (2.5 µL P40 + 1.5 µL AgeI + 1.5 µL NgoMIV + 3 µL CutSmart + 21.5 µL ddH2O)
- Incubation over night at 37°C
Generation of GFP expressing E. coli cells
Investigator: CG, JH
Aim of the experiment: Various inductor concentrations
Procedure:
- 12x 5 ml LB+Cam media were inoculated with 50 µl preculture
- when cultures reached OD550 of 0.5, they were induced with tetrazycline-anhydride of various concentrations (0 ng/µl, 20 ng/µl, 40 ng/µl etc., 200 ng/µl)
- t=0, =30, =90, =150, =210, =270 a 100 µl aliquot of each culture was stored at 4 °C in the refrigerator
Dialysis of biotinylated eGFP
Investigator: JH, JB
Aim of the experiment: Purification of biotinylated eGFP
Procedure:
- eGFP was poured in a dialysis hose (cut-off 14 kDa)
- The hose was then placed in 2 L ice cold Tris/HCl 20 mM pH 8.0
- The dialysis took place at 4°C over night
Ligation of F17.1 (p(cat)) and F18 (stron RBS) and transformation in XL1 blue
Investigator: JH
Aim of the experiment: Ligation and transformation in XL1blue
Procedure:
- Plasmid was ligated with the T4 DNA Ligase following the manufacturer’s protocol (NEB)
- 9 µL F18 (B0034 in pSB1A2; RBS) and 1 µL of F17.1 (I14033; P(cat)) were used [AmpR]
- The Ligation mix was incubated for at least 2h at RT
- 7 μL Ligation mixed were used for the transformation of 50 μL XL1 blue
- Transformation was performed following the SOP [AmpR], incl. rescue plate
Friday, June 3th
Cloning of F8 (A3C5), F9 (Avi-Tag) and F14 (EspP) into F20
Investigator: CR
Aim of the experiment: Cloning of A3C5, Avi-Tag and into pSB1C3
Procedure:
- FastAP was added and the digestion of F20 and was incubated for another 15 min at 37°C
- The digestion was purified by gelelctrophoresis (1%, 75V, 1h)
- Plasmid backbone (signal 1) was cut from the gel
- Gelextraction was performed after manufacturer's protocol (Gelextractionkit, Qiagen)
Results:
Signal 1: F20
[F20]= 13,8 ng/μL
Generation of GFP expressing E. coli cells
Investigator: CG, NA
Aim of the experiment: SDS PAGE for verification of successful GFP expression
Procedure:
- SDS electophoresis according to SOP
- Lane 1: #12, 90 min
- Lane 2: #12, 150 min
- Lane 3: #12, 210 min
- Lane 4: #12, 270 min
- Lane 5: 8 µl
Results:
Inoculation of colonies from F19 (pcat-strong RBS)
Investigator: CG
Aim of the experiment: three colonies were picked from yesterdays transformation
Procedure:
- 3x 5 ml LB+Amp media
- Each culture was inoculated with one colony
- Incubation at 37°C overnight
Cloning of F8 (A3C5), F9 (Avi-Tag) and F14 (EspP) into F20 (pSB1C3)
Investigator: CG
Aim of the experiment: Ligation of F21, F22 and F23 into F20 and transformation
Procedure:
- Ligation was performed according to the SOP with: 0.7 µl F8 (1:10) / 9.3 µl F20, 0.7 µl F9 (1:10) / 9.3 µl F20, 9.1 µl F14 / 0.9 µl F20
- Transformation was performed according to the SOP on LB Cam-Agar
Precipitation of Streptavidin
Investigator: NA, JB, LK
Aim of the Experiment: Purification of Streptavidin
Procedure:
- Slowly add (NH4+)2SO42- until the concentration reaches 40% (23,1 g/100mL) while stirring
- Let it stir over night to ensure complete precipitation
- Centrifuge at 8500 rpm at 4°C for 15min (rotor: SLA1500), dismiss the pellet and use the supernatant for further processing
- Slowly add (NH4+)2SO42- until the concentration reaches 70% (19,1 g/100mL) while stirring
- Let it stir over night (slowly to avoid foaming)
- Centrifuge at 8500 rpm at 4°C for 15min (rotor: SLA1500), dismiss the supernatant and use the pellet for further processing
- Resuspend the protein pellet in a minimum volume of TRIS/HCl; pH 8; 20mM (NO SALT) (6 mL per pellet), the pellets where then united and titrated with Buffer until the solution became clear. Total amount of buffer: 40mL
- Transfer to 50 mL Falcon Tubes and centrifuge at full speed for 15 min. Filter the supernatant through 45nm sterile filter and keep the pellet (rescue).
Saturday, June 4th
Inoculation of colonies from F21 (pSB1C3 (A3C5)), F22 (pSB1C3 (Avi-Tag)), F23 (pSB1C3 (EspP))
Investigator: NA
Aim of the experiment: three colonies were picked from each transformation
Procedure:
- 3x 5 ml LB+Cam media for each fragment
- Each culture was inoculated with one colony
- Incubation at 37°C overnight
MiniPrep of F19 (pSB1A2 (pcat-strong RBS))
Investigator: NA
Aim of the experiment: Extraction of Ligation: F17+F18 (starke RBS+P(cat))
Procedure: MiniPrep was performed after manufacturer's protocol (QIAprep MiniPrep, Qiagen) Concentrations: K1= 66,2 ng/µl; K2= 60,2 ng/µl; K3= 104,4 ng/µl
Week 4 (June 6th - June 12th)
Monday, June 6th
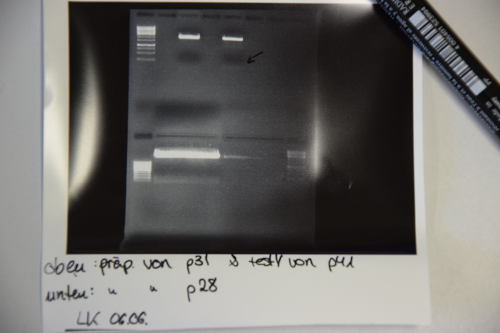
Cloning of TetR from p28 into p31 (pSB1C3(weak RBS))
Investigator: LK, JH, NA
Aim of the experiment: Cloning TetR in front of weak RBS in pSB1C3
Procedure:
- Digestion of p28 with EcoR1 and Spe1 (20µl DNA, 2,5 µl each enzyme, 5µl CutSmart buffer, 20µl ddH2O) and p31 (4µl DNA, 1µl EcoRI / XbaI, 2µl, 12µl DNA) -> Incubation at 37°C for 2h.
- Electrophoresis: 1% Agar, 15 min , 70 V (oben p31 und p41, unten p28)
- Gelextraction concentrations: p28 (F25) = 2,3 ng/µl
p31 (F24) = 7,8 ng/µl
- Ligation with 6,3 µl vector (F24) and 1,7 µl Insert (F25) + 1µl Buffer + 1µl Ligase over night, 16°C
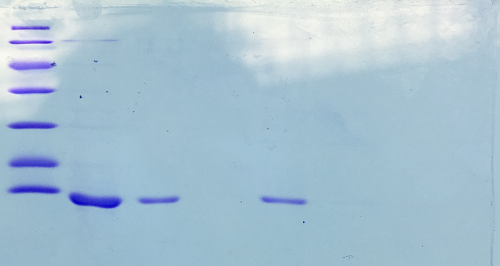
SDS-PAGE of Streptavidin
Investigator: NA, JH, LK
Procedure: mixing of 80 µl sample with 20 µl SDS buffer and heating at 95°C for 10 min. 1 h staining, 1 night unstaining. Concentration of streptavidin was measured via UV/VIS spectroscopy.
c = 23,5 mg/ml
Result:
- 1. Lane: 8 µl Marker
- 2. Lane: 5 µl of Streptavidin_1:10
- 3. Lane: 5 µl of Streptavidin_1:50
- 4. Lane: 5 µl of Pellet_1:500
- 5. Lane: 5 µl of after 40% precipitation_1:10
- 6. Lane: 5 µl of supernatant after 70% precipitation and centrifugation
Successful Streptavidin production verified.
Sequencing of P41 (pSB1C3 (strongRBS-p(cat)))
Investigator: NA
Aim of the experiment: Sequencing of P41
Procedure: Sequencing batches were prepared after manufacturer's protocol (15 µL plasmid DNA (100 ng/ µl) and 2 µL sequencing primer (VF2))
The plasmids prepared received the following barcode:
- P41 : FR11326633
Tuesday, June 7th
Transformation of P30 (Tet repressed GFP), P36 (Tet repressed RFP), P37 (Tet repressed YFP)
Investigator: NA
Aim of the experiment: Transformation of RFP, GFP and YFP in BL21
Procedure: transformation in competent E. Coli BL21 according to protocol + rescue
Result: plates (LB Cam) in incubator for further processing (37 °C)
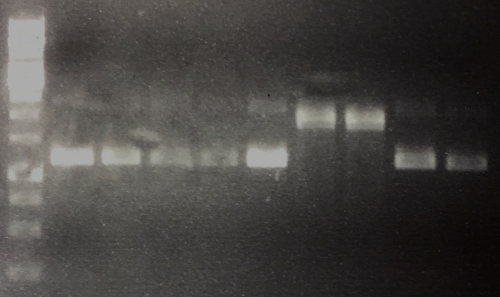
MiniPrep of P42 to P50, analytic digestion, sequencing
Investigator: CG
Aim of the experiment: Verification of successful cloning
Procedure: MiniPrep was performed according to manufacturer's protocol (QIAprep MiniPrep, Qiagen)
- Concentrations (ng/µl each) for P42: 260, P43: 247, P44: 181, P45: 118, P46: 261, P47: 135, P48: 148, P49: 240, P50: 299
- Analytic digestion: 0.3 µl AgeI, 2 µl CutSmart, 6.7 µl H2O for P42, P43, P44, P45, P46, P49, P50 and 0.3 µl EcoRI, 0.3 µl PstI, 2 µl CutSmart, 6.4 µl H2O for 2 h at 37°C
- 1% agarose gel electrophoresis
- Sequencing: P42= FR11326632 (VF2), P45 =FR11326631 (VF2)
Results:
- Lane 1: 5 µl 1 kb Ladder
- Lane 2-10: Digestion of P42 to P50. P47 and P48 are carrying the insert. All other plasmids are religated and lost their AgeI site, hence they run in the ccc position.
Dialysis of not biotinylated eGFP
Investigator: NA
Aim of the experiment: Purification of eGFP for mass determination
Procedure:
- eGFP was poured in a dialysis tubes
- The tubes were then placed in ice cold ammonium acetate
- The dialysis took place at 4°C over night
Polymerisation attempts
Investigator: JH, CR, CG, JB
Aim of the experiment: Identification of optimal polymerisation conditions for biotinylated eGFP and BSA in a Streptavidin reservoir
Procedure:
- variate of compositions for both solutions was examined to modify density properties during “printing”
- all compositions can be found in excel sheet
Results:
- no or little successful polymerization at Streptavidin concentrations of 4 mg/mL and below; immense density incompatibilities
- sucessfull polymerization at Streptavidin concentrations of 10 mg/mL and BSA solution with approx. 15% glycerol
- no success with biotinylated eGFP (too few Biotin molecules?!)
Wednesday, June 8th
Sequencing of P36 (Tet repressed RFP) and P37 (Tet repressed YFP)
Investigator: NA
Aim of the experiment: Sequencing of P36 and P37 in both directions
Procedure: Sequencing batches were prepared after manufacturer's protocol (15 µL plasmid DNA (50-100 µM) and 2 µL sequencing primer)
The different plasmids we prepared received the following barcodes:
- P36 (VF2): FR11326630
- P36 (VR): FR11326629
- P37 (VF2): FR11326628
- P37 (VR): FR11326627
Dialysis of Streptavidin
Investigator: NA, JH
Aim of the experiment: Purification of Streptavidin (possible ammonium sulfate contamination after precipitation)
Procedure:
- Streptavidin was poured in a dialysis hose (cut-off 14 kDa)
- The hose was then placed in 3 L ice cold Tris/HCl 20 mM pH 8.0 (no salt)
- The dialysis took place at 4°C over night
Polymerisation attempts
Investigator: JH, JB
Aim of the experiment: Identification of optimal polymerisation conditions for biotinylated BSA in a Streptavidin reservoir
Procedure:
- variate of compositions for both solutions was examined to modify density properties during “printing” (Streptavidin at 10 mg/mL or higher)
- all compositions can be found in excel sheet
Thursday, June 9th
Generation of GFP, YFP, RFP expressing E. coli cells
Investigator: CG
Aim of the experiment: Various inductor concentrations, various fluorescent proteins were expressed in E coli cells
Procedure:
- 3x 5 ml LB+Cam media were inoculated with 50 µl preculture of GFP (P30), RFP (P36), YFP (P37)
- when cultures reached OD550 of 0.5, they were induced with anhydro-tetrazycline of various concentrations (0 ng/µl, 100 ng/ml, 200 ng/ml)
- 50 ng/ml = 12,5 µl of 1:100 2 mg/ml stock solution, 200 ng/ml = 50 µl of 1:100 2 mg/ml stock solution
- t=0, =4 h and stored on ice
Resuts: no fluorescent could be detected. Therefore an overnight induction at 37°C with 1 µM/ml inductor concentration was performed with the RFP plasmid.
Biotinylation of GFP expressing E. coli cells
Investigator: CG
Aim of the experiment: Biotinylation of P30 carrying E. coli cells
Procedure:
- 850 µl P30 preculture from Thursday 2nd were centrifuged and the supernatend was discarded
- Pellet was resuspended in 800 µl PBS-buffer
- 100 µl 100 mM Biotin-NHS was added
- incubation for 4 h at room temperature
- Experimenting with some glycerol and streptavidin concentrations. A more structured approach will be done the next day.
Results: A shape thin “sausage” could be detected. Therefore a bigger E. coli culture was biotinylated at 4°C overnight for experimenting with the next day.
Cloning of TetR promotor and weak RBS in pSB1C3
Investigator: CR, JB
Aim of the experiment: MiniPrep and NanoDrop of F26 K1, K2 and K3 Sequencing of P51
Procedure: MiniPrep was performed according to manufacturer's protocol (QIAprep MiniPrep, Qiagen) Concentrations were as following:
- P51: 163,3
- P52: 151,7
- P53: 140,5
P51 was sequenced:
- 1. with VF2: FR11326626
- 2. with VR: FR11326625
Biotinylation of eGFP
Investigator: VL, JB
Aim of the experiment: Biotinylation of eGFP with 200x biotin-NHS molar excess over eGFP (15x excess over surficial K residues). NB: previous trial unsuccessful. Excess of biotin was likely insufficient to ensure efficient biotinylation, esp. since biotin-NHS slowly hydrolyzes in the aqueous environment.
Procedure: 1 ml of eGFP was biotinylated with an 200x excess of biotin in DMF (100 uM; 38 uL), 5.5 h at rt. The solution was dialysed versus 20 mM Tris/HCl pH 8 overnight at 4 °C.
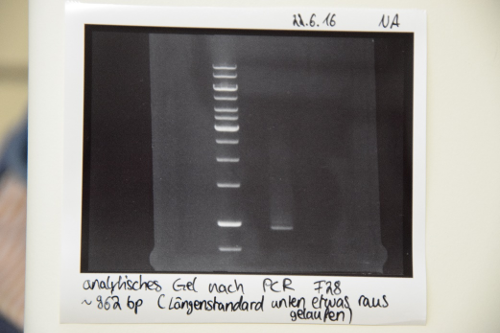
PCR of gene syntheses
Investigator: LK
Aim of the experiment: Amplification od iGEM_1_SA-m1 (F27), iGEM_1_mRuby_EGFR (F28) and iGEM_2_IRES-BirA (F29)
Procedure:
- Add 100µl of ddH2O to the gene sythesis samples and incubate for 20 min at 50°C
- Dilute samples to a final concentration of max. 1ng/µl (1:10) with water.
- Approach:
1µl DNA (diluted)
5µl buffer
0,5µl dNTPs
1,25µl forward Primer VF2
1,25µl reverse Primer VR
0,25µl Q5 Polymerase
Fill up with water up to 25µl
- Make sure to pipette everything on ice and add the polymerase at the end
- Setup: (calculated with NEB calc. http://tmcalculator.neb.com/#!/)
98°C_1min initiation step
98°C_10sec
66°C_30sec > step 2-4: 35 repeats
72°C_30sec
72°C_2min final step
4°_hold
- The samples were then prepared with the PCR Product Purification Kit
Polymerisation experiments for biotinylated eGFP and BSA with Streptavidin
Investigator: JH, CR, JB
Aim of the experiment: Determination of ideal concentrations for both protein interaction partners as well as buffer viscosity (glycerol content etc.) for polymerisation in a test tube
Procedure:
- Different concentrations of proteins and glycerol were tested to support stable polymerisation without protein aggregates rising to the surface or sinking to quickly
- The experiments done are not going to be further documented as the streptavidin used for the experiments was previously determined in a wrong fashion and was actually considerably lower than expected (4x lower). Streptavidin was, due to that circumstance, concentrated to be able to support experiments with at least 5 mg/ml concentrations
- First experiments with concentrated streptavidin seemed promising, though a complete series of experiments with varying parameters should be conducted in the future
Sequencing of P29 (pSB1C3 (double terminator), P36 (pSB1C3 (Tet repressed RFP)) and P37 (pSB1C3 (Tet repressed RFP))
Investigator: JB
Aim of the experiment: Sequencing of P29, P36 and P37
Procedure: According to the manufacturer's protocol.
- P29: FR11326624 (VF2)
- P36: FR11326623 (VF2)
- P37: FR11326621 (VR), FR11326622 (VF2)
Inoculation of (pre)cultures for a pSB1C3 midiprep
Investigator: JB, VM
Aim of the experiment: Massively amplifying the pSB1C3 vector (which will be needed for building up new biobricks) by midiprepping the P40 plasmid (RFP-generator in pSB1C3). Similar to minipreps, but on a greater scale. Procedure: 3 ml precultures were inoculated with a clone from the plate previously used for minipreps. After the precultures had grown for 4 hours (due to shortage of time), 2 x 50 ml cultures were inoculated and shaken overnight at 37°C. The next day, cultures were put into the fridge for storage until Monday.
Week 5 (June 13th - June 19th)
Monday, June 13th
Digestion of gene synthesis iGEM1 and iGEM2
Investigator: LK, JH
Aim of the experiment: Digest F28 (iGEM1) and F29 (iGEM2) in order to ligate them into P40, which was equally digested (to get rid of the rfp-generator and have pSB1C3 as backbone)
Procedure:
- 30µl of F28 were digested for 3h with SpeI and NgoMIV, and 30 µl of F29 were digested with XbaI and PstI
- 2 x 20 µl where digested with the equivalent enzymes for 3h
- After electrophoresis the bands were cut out and purified
Results: Results show that PCR of F28, F29 might not have worked. P40 was extracted from gel (F30 and F31). PCR of gene synthesis was repeated (50µl!)
Midiprep of P40
Investigator: LK
Aim of the experiment: Purification of P40 from
Procedure:
- See protocol of Midiprep kit
- 1xuse-centrifugation-columns broke in centrifuge during last step, DNA rescue was tried by resuspending rest of the solution in the tube in EB buffer and redo the last steps (isoprop and ethanol precipitation)
- Airdry "pellet"(no pellet visible) for 15 min under fume hood and resuspend in 100µl EB Buffer
- Measure concentration with nanodrop
Preparation of Collagen + Biotinylation
Investigator: VL, JL
Aim of the experiment: prepare collagen solution + biotinylate small sample
Procedure:
- Dissolve 5 g collagen in 50 mL Tris/HCl pH 8 20 mM w/o salt --> 770 uM solution (100 mg/mL)
- Sterile filter
- Biotinylation: 980 uL Tris buffer + 20 uL collagen solution + 30 uL biotin-NHS (100 mM in DMF, 200x molar excess over collagen monomers)
- Reaction time:
- Dialyze 4 °C Tris/HCl pH 8 20 mM w/o salt o/n
Transformation of P9 (pASK75)
Investigator: JL, VL
Aim of the experiment: transform streptavidin expression vector P9 (clone 4) into E. coli BL21
Procedure:
- Standard (see booklet): 45 s at 42 °C; incubate 1h, 37°C; plate: medium w. chloramphenicol (+ rescue plate) Incubate o/n 37°C.
- Wrong antibiotic plate, repeated the next day of the experiment: Massively amplifying the pSB1C3 vector (which will be needed for building up new biobricks) by midiprepping the P40 plasmid (RFP-generator in pSB1C3). Similar to minipreps, but on a greater scale.
Tuesday, June 14th
Mass spectrometry of biotinylated eGFP
Investigator: CR
Aim of the experiment: identify biotinylation degree of eGFP
Procedure:
[generic_display_report] (i could not upload this file, sorry.)
Results: about 4-5 biotins per protein. To verify the biotinylation degree the unbiotinylated eGFP will be measured.
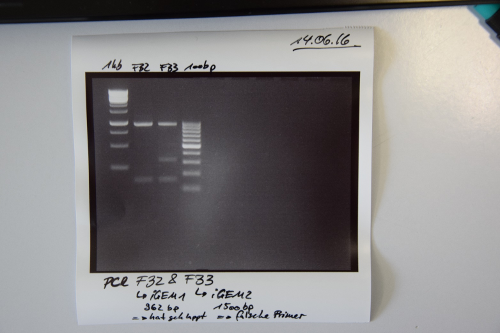
Cloning of F32 (PCR iGEM_1 mRuby-EGFR) and F33 (PCR iGEM_2 IRES-BirA) in pSB1C3
Investigator: CR
Aim of the experiment: Purification of PCR products
Procedure:
- Volume of PCR reaction: 50 µL
- PCR products were purified by manufacturers protocol (QIAquick PCR purification kit, Qiagen)
- Concentration measured by Nanodrop:
- F32: 87,5 ng/µL
- F33: 68,3 ng/µL
- Test if PCR worked:
- Samples were applied on a 2% agarose gel and was run at 80 V for 40 min.
Results:
- PCR of F28 worked --> F32
- PCR of F29 did not work --> wrong primers were used for amplification --> F33 not right (Re-Do PCR with appropriate primers
Cloning of F32 (PCR iGEM_1 mRuby-EGFR) and F33 (PCR iGEM_2 IRES-BirA) in pSB1C3
Investigator: CG
Aim of the experiment: Digestion of F32 (SpeI, NgoMIV) and F33 (XbaI, PstI)
Procedure:
- Digestion F32: 27 µl of F32 (2.4 µg), 5 µl CutSmart Buffer, 1.5 µl SpeI, 2 µl NgoMIV, 14.5 µl H2O
- Digestion F33: 27 µl of F32 (1.8 µg), 5 µl CutSmart Buffer, 1.5 µl XbaI, 1.5 µl PstI, 15 µl H2O
- Incubation for 3 h at 37°C
- Preperative gel:
- Gelextraction of F32 upper band and F32 lower band was executed by manufacturers protocol (Qiagen)
- F32 upper band (mRuby) was named F34 (=10 g/yl)
- F32 lower band (eGFR) was named F35 (=6 ng/yl)
Cloning of F32 (PCR iGEM_1 mRuby-EGFR) and F33 (PCR iGEM_2 IRES-BirA) in pSB1C3
Investigator: CR
Aim of the experiment: Ligation of F34 (mRuby) and F35 (eGFR) in F30 (pSB1C3); Tranformation in XL1blue
Procedure:
- Ligation mixtures were prepared as following:
- F34: 8.0 μL F34, 2 μL F30, 2 μL Ligase buffer, 7 μL ddH2O, 1 μL Ligase)
- F35: 7.6 μL F35, 2.4 μL F30, 2 μL Ligase buffer, 7 μL ddH2O, 1 μL Ligase)
- Ligation was incubated for 30 min at RT
[something is missing here]
Re-Sequencing of P37 (Tet repressed YFP)
Investigator: CG
Aim of the experiment:
Purification and sequencing of P30
Procedure:
- P30 was heated up to 100 °C and centrifugated afterwards to remove proteins from Promotor (if there are some), the supernatant is sequenced
- Sequencing batches were prepared after manufacturer's protocol
- (15 μL plasmid DNA (50-100 μM) and 2 μL sequencing primer)
- The plasmid we prepared received the following barcode: P37 (VF2) : FR11326620
Streptavidin expression
Investigator: CR
Aim of the experiment: Transformation of P9 (pASK74 (SA1)) in BL21
Procedure:
- Transformation was executed following SOP
- Transformed cells were plated on LB medium (Amp) (50 µL and rescue (50 µL)
Dialysis of Streptavidin and biotinylated BSA
Investigator: CR
Aim of the experiment: Streptavidin and bitinylated BSA are both in Tris/HCl without salt. SInce we will perform later experiments in salty solutions the dialysis was performed against 137 mM NaCl (Saltconcetration of PBS)
Procedure:
- 3 L Tris/HCl NaCl solution was prepared
- Dialysis was prepared according to SOP
- Cut off Dialysis hose: 14 kDa
Inoculation of (pre)cultures of P40 for a pSB1C3 midiprep [repeat]
Investigator: JH
Aim of the experiment: Massively amplifying the pSB1C3 vector by midiprepping the P40 plasmid (RFP-generator in pSB1C3)
Procedure: four 4 ml precultures were inoculated with a clone from the plate previously used for minipreps. After the precultures had grown for 6 hours, 50 ml cultures were inoculated and shaken overnight at 37°C.
Subcloning of P41 (strong RBS-p(cat)) Insert into pSB1C3
Investigator: JH
Aim of the experiment: Moving P41 (pSB1A2; Pcat+weakRBS) to pSB1C3 for biobrick submission
Procedure:
- Digestion of P41 with XbaI and PstI (10 µl DNA, 1,5 µl each enzyme, 3 µl CutSmart buffer x10, 14 µl ddH2O) -> Incubation at 37°C for 2h.
- Electrophoresis: 2% Agar, 40 min , 90 V (1kb ladder, empty, P41, 100b ladder)
- GelExtraction
Resuts: no visible signal of insert at approx. 100b. Area was cut blind for GelEx. No DNA in the sample according to NanoDrop measurement. Repeated the next day.
Inoculation of colonies from NanoLuc
Investigator: JH
Procedure:
- 2x 4 ml LB+Cam media
- Each culture was inoculated with one colony
- Incubation at 37°C overnight
Wednesday, June 15th
Subcloning of P41 (strong RBS-p(cat)) Insert into pSB1C3
Investigator: CG
Aim of the experiment: Moving P41 (pSB1A2; Pcat+weakRBS) to pSB1C3 for biobrick submission. No gel extraction was performed because the fragement is too small to visualize on the gel.
Procedure:
- Digestion of P41 with XbaI and PstI (10 µl DNA, 1,5 µl each enzyme, 3 µl CutSmart buffer x10, 1 µl FastAP, 13 µl ddH2O) -> Incubation at 37°C for 2 h, heat inactivation at 80°C for 20 min.
fragement is to small to visualize on agel.
- Subcloning into F31: 3.8 μL F38, 6.2 μL F31, 2 μL Ligase buffer, 7 μL ddH2O, 1 μL Ligase according to the SOP
- Transformation into E. coli XL1 according to the SOP
Miniprep of pSB1C3(Nanoluc)
Investigator: LK
Aim of the experiment: Preperation of a BioBrick with NanoLuciferase in pSB1C3
Procedure:
- The Miniprep was done according to the protocol.
- The two tubes where pooled.
- The new Plasmid is found under P54 and was sent to sequencing.
Polymerisation
Investigator: EU
Aim of the experiment: Determine optimal polymerisation conditions for BSA and Collagen
Procedure: The experiment was performed in a 96 Well plate:
Results: maximum concentration of Streptavidin and biotinylated Protein works best. Collagen does not work. Glycerolconcentrations do not inhibit interaction. Reaction might be to slow at given concentrations to be effective in printing process.
MidiPrep of pSB1C3 with RFP generator (P4)
Investigator: NA
Aim of the experiment: purification of pSB1C3 with RFP generator for further processing (P56)
Procedure:
- MidiPrep was performed according to manufacturer's protocol (QIAprep MidiPrep, Qiagen)
Results: Concentration very low (12ng/μL), but high volume (500 μL)
Thursday, June 16th
MiniPrep of F36 (Ligation mRuby in pSB1C3) K1 & K2, F37 (Ligation EGFR in pSB1C3), I13453 (pBad promotor) K1 & K2, K577881 (pC+AraC+pBad+GFPc) K1 & K2
Investigator: CR
Aim of the experiment: isolate Plasmids
Procedure:
- MiniPrep was performed according to manufacturer's protocol (QIAprep MiniPrep, Qiagen)
- Plasmids were named as following: [concentration]
F36 K1: P57 [212,9 ng/μL]
F36 K2: P58 [181,3 ng/μL]
F37: P59 [195,5 ng/μL]
K577881 K1: P60 [557,2 ng/μL]
K577881 K2: P61 [791,9 ng/μL]
I13453 K1: P62 [130 ng/μL]
I13453 K2: P63 [460 ng/μL]
Sequencing of P29 (double terminator), P36 (Tet repressed RFP) and P37 (Tet repressed YFP)
Investigator: JB
Aim of the experiment: Sequencing of P56, P57, P59, P61 and P62
Procedure: According to the manufacturer's protocol.
- P56: FR11326618 (VF2)
- P57: FR11326617 (VF2)
- P59: FR11326616 (VF2)
- P61: FR11326613 (VR), FR11326615 (VF2)
- P63: FR11326614 (VF2)
MidiPrep P4 (pSB1C3)
Investigator: VL
Aim of the experiment: isolate Plasmids
Procedure:
- MidiPrep was performed according to manufacturer's protocol (QIAprep MidiPrep, Qiagen). Centrifugation steps at 20,000 g performed at 12,500 rpm in special centrifugation tubes. Since only one column was left, both prepped cell pellets were applied to one column. Flow-through and wash was collected.
- Conc:
Amplification of BioBricks (overnight cultures for minipreps)
Investigator: JB
Aim of the experiment: Inoculation of 5 ml overnight cultures for minipreps. The following biobricks were used:
- BBa_K157012
- BBa_K243004
- BBa_K243006
- BBa_K157001
- BBa_K243005
- BBa_I712009
- BBa_K404108
- BBa_K782061
- BBa_K1371012
- BBa_K782060
Procedure:
- Three hours of incubation of the tubes containing the bacterial agar at 37°C (Note: 12 hours are actually recommende)
- Inoculation of two times 5 ml preculture containing the appropriate antibiotic per clone. For KanRAmpR (reistance against both ampicillin and kanamycin) it was only selected for ampicillin.
- Incubation shaking overnight at 37°C
PCR Amplification of genesynthesis 2 (F29; IRES-BirA)
Investigator: LK
Aim of the experiment: Amplification of Genesynthesis_2 (IHRES_BirA_StrepTag)
Procedure: A 50µl approach was made:
- 33,5 µl Water
- 2µl DNA (F29, diluted 1:10)
- 2,5µl of each Primer (O11 and O12 c:10µM)
- 0,5µl dNTPmix
- 5µl Reaction buffer
- 0,5µl Q5-Polymerase
- The Annealing Temp. was set an 58°C
- Next step is to run an analytic gel (band should be visible at 1,5 kB)
Friday, June 17th
Cloning of genesynthesis 2 (IRES-BirA) into pSB1C3
Investigator: CG, NA, JH
Aim of the experiment: Purification oft he PCR reaction, gel verification, restriction digestion (F29), ligation with F31 and transformation
Procedure:
- Yesterdays PCR was purified according to Qiagen PCR purification kit
- An anlaytic gel electrophoresis was performed on a 1% gel with 4 µl purified PCR product
- Restriction digest: 26 µl purified PCR product, 1.5 µl XbaI, 1.5 µl PstI, 5 µl NEB SmartCut, 16 µl H2O. Incubation for 2 h at 37°C.
- Gel extraction
- Ligation: F31
- Transformation
Results: Gel electrophoresis after PCR purification exhibit a thin signal at 1.5 kb. Next time 1:30 min for elongation time might increase the signal.
Miniprep new iGEM BioBricks
Investigator: VL
Aim of the experiment: Purify plasmid DNA from transformed cells
Procedure:
- Miniprep according to manual (pellet centrifuged 5000 rpm, 10 min, 4 °C)
- Elution with 50 uL EB Buffer
- Concentrations (all ng/uL):
K782061 (a) 172.9, (b) 53.0
K243006 (a) 241.1, (b) 58.6
K1371012 (a) 138.0, (b) 43.0
K157001 (a) 184.3, (b) 186.4
K782060 (a) 339.0, (b) 254.0
K243005 (a) 62.3, (b) 176.2
K243004 (a) 122.3, (b) 110.3
I711009 (a) 185.2, (b) 174.7
K157012 (a) 181.2, (b) 195.3
K404108 (a) 116.4, (b) 142.5
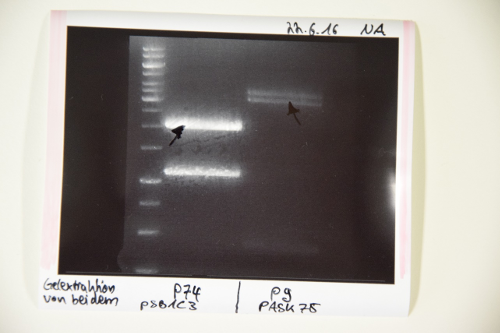
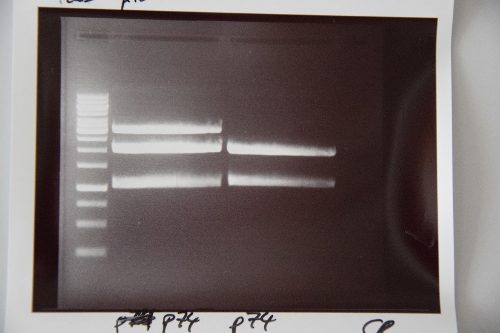
MidiPrep of pSB1C3 with RFP generator (P4)
Investigator: NA
Aim of the experiment: purification of pSB1C3 with RFP-Reportercasette for further processing (P74)
Procedure: MidiPrep was performed according to manufacturer's protocol (QIAprep MidiPrep, Qiagen)
Results:
Cloning of IRES-BirA (F33->F40) into digested F31 (pSB1C3)
Investigator: JH, LK
Aim of the experiment: Ligation of IRES-BirA (F29->F33->F40) into F31 (digested P40 by XbaI and PstI = pSB1C3 without insert), gelelectrophoresis and tranformation in XL1blue
Procedure:
- F33 (PCR product of F29/gen synthesis 2) was digested with XbaI and PstI
- Gelextraction of band at 1500 bp was executed by manufacturers protocol (Qiagen)
- intermediate result: F40 with 6.6 ng/µL
- Ligation mixtures were prepared as following:
- 9.6 µL F40, 0.4 µL F31, 2 μL Ligase buffer, 7 μL ddH2O, 1 μL Ligase
- Transformation: (Saturday)
- Transformation of Ligation (F29+F31) into XL-1 blue was done according to the SOP. The plate was incubated until Sunday at 37°C.
- 3mL-cultures in LB+ 30µg/ml cam where inoculated on Sunday
- Mini-prep according to manufacturer's protocol was done on Monday.
PCR, purification and digestion of P19 (EspP) and F28 (mRuby EGFR) for BioBrick creation
Investigator: JB, LK
Aim of the experiment: Amplification of EspP from P19 and mRuby/EGFR (F28) for creation of Biobricks
Procedure (for both fragments):
- PCR according to the SOP (primers for P19: O13 and O03, primers for F28: VR and VF2)
- PCR purification via the purification kit according to the manufacturer's protocol (concentrations: P19(EspP):119,5 ng/µl F28(mRuby):56,2ng/µl
- Analysis on an 1% agarose gel using 5 µl purified PCR product
- Restriction digestion with NgoMIV/SpeI (for both cases)
- 40µl DNA
- 1,5µl of each Enzyme
- 2µl ddH2O
- 5µl CutSmart Buffer
- The tubes where incubated at 37°C for ~2 days.
Results: Analytical gel electrophoresis after PCR purification shows thick bands at roughly 1000 bp
Still to do:
- Gel extraction of both signals for F28 and one signal for EspP-PCR
- Ligation with appropriate vector (e.g. F30)
- Transformation
Minipreps of F39 (strong RBS-pcat in pSB1C3)
Investigator: JB
Aim of the experiment: Amplification of F39 (ligation, pCat with strong RBS)
Procedure:
- Miniprep according to the manufacturer's protocol
- Resulting plasmid: P76
- Concentration: 52 ng/µl
Minipreps of pSB1C3 with RFP generator (P4)
Investigator: JH
Aim of the experiment: purification of pSB1C3 with RFP-Reportercasette for further processing (P75)
Procedure:
- Minipreps according to the manufacturer's protocol (with 8* 6 mL culture)
- Resulting plasmid: P75
- Concentration: 142.2 ng/µl [~200 µL]
Inoculation of colony from NanoLuc [P54, Repeat]
Investigator: JH
Procedure:
- 5 ml LB+Cam media
- culture was inoculated with one colony
- Incubation at 37°C overnight
Saturday, June 18th
Miniprep of P54 (NanoLuc)
Investigator: LK
Aim of the experiment: Purification of P54 (NanoLuc)
Procedure:
- Miniprep according to the manufacturer's protocol
- Resulting plasmid: pSB1C3 mit NanoLuc (P54, von )
- Concentration: 497,9 ng/µl
Hybridisation of O19 and O20 (StrepTag)
Investigator: LK
Aim of the experiment: Hybridisation of the Strep-Oligos
Procedure: 2µl of each Oligo where diluted in 16µl ddH2O and incubated for 10 min. at 95°. Cooling down at RT for at least half an hour. Stored in fridge
Week 6 (June 20th - June 26th)
Monday, June 20th
Miniprep of F42 (IRES-BirA in pSB1C3)
Investigator: LK
Aim of the experiment: Purification of IRES-BirA Plasmids
Procedure:
- Miniprep according to the manufacturer's protocol
- Resulting plasmid: pSB1C3 with IRES-BirA
Next Steps:
- analytical digestion with EcoRI+PstI and gel (expected bands at 1500 and 2000 Bp)
- Sequencing
Results: Concentration: 583,5 ng/µl
PCR and Purification of BirA
Investigator: LK, JB
Aim of the experiment: Amplification of BirA-construct
Procedure:
- PCR according to the SOP (primers: O17 and O18)
- PCR purification via the purification kit according to the manufacturer's protocol
- Analysis on an 1% agarose gel using 5 µl purified PCR product
Results:
- Concentration: 185,8 ng/µl --> F41
- Analytical gel shows no significant band at 960 Bp -->Repeat
Next Steps:
- Repetition of PCR and analytical gel!
Gel and Gelextraction of P19 (QC EspP) and F28 (EspP and mRuby)
Investigator: LK
Aim of the experiment: Purification of both constructs for Ligation
Procedure:
- Preperative gel according to SOP
- The Purification was done according to the manufacturer's protocol. The EGFR gel-fragment was eluted in 50µl and the mRuby-fragment in 30µl (because of thin band)
Results:
- One thick band just before 1000Bp and one lighter band just before 750 Bp. This fits to the lengths of EspP and mRuby.
- Concentrations: EspP(now F43): 49,5ng/µl mRuby(now F44): 9,9ng/µl
Next Steps:
- Ligation into pSB1C3
Sequencing of F42 (pSB1C3(IRES-BirA)), P54 (pSB1C3(NanoLuc)) , P74 (pSB1C3(RFP generator)) and P76 (pSB1C3(strong RBS-pCat))
Investigator: JL
Aim of the experiment: Sequencing of F42, P54, P74 and P76 in forward direction.
Procedure: Sequencing batches were prepared after manufacturer's protocol (15 µL plasmid DNA (50-100 µM) and 2 µL sequencing primer)
The different plasmids we prepared received the following barcodes:
- F42 (VF2): FR11326601
- P54 (VF2): FR11326600
- P74 (VF2): FR11326599
- P76 (VF2): FR11326598
PCR and Purification of genesynthesis 6 (iGEM6) Streptavidin/Traptactin
Investigator: NA
Aim of the experiment: Amplification of gen synthesis 6
Procedure: PCR according to the SOP (primers: VF2 and VR)
Next Steps:
- PCR purification via the purification kit according to the manufacturer's protocol
- Analysis on an 1% agarose gel using 5 µl purified PCR product
- PCR purification via the purification kit according to the manufacturer's protocol
- Analysis on an 1% agarose gel using 5 µl purified PCR product
PCR and Purification of BirA (F41)
Investigator: NA
Aim of the experiment: Amplification of BirA (F29)
Procedure: PCR according to the SOP (primers: O17 and O18)
Next Steps:
- PCR purification via the purification kit according to the manufacturer's protocol
- Analysis on an 1% agarose gel using 5 µl purified PCR product
- PCR purification via the purification kit according to the manufacturer's protocol
- Analysis on an 1% agarose gel using 5 µl purified PCR product
--> repeat with gene-synthesis 2 (F29 may not be the gene-synthesis 2)
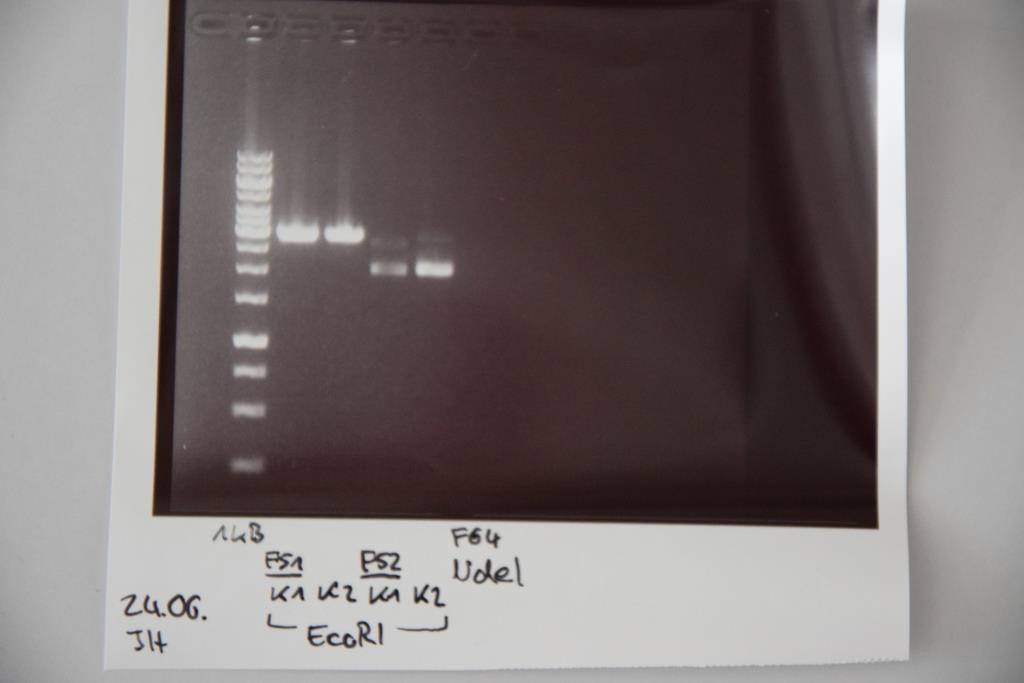
Ligations of StrepTag, EspP and mRuby3 with F30 (digested pSB1C3)
Investigator: JH
Aim of the experiment: Ligations of StrepTag (O19+O20), EspP (F43) and mRuby3 (F44) into F30 (NgoMIV and SpeI; pSB1C3 without insert)
Procedure:
- Ligation mixtures were prepared as following:
- O19+O20: 1.7 (O19+O20), 8.3 μL F30, 2 μL Ligase buffer, 7 μL ddH2O, 1 μL Ligase
- F43: 4.6 μL F43, 5.4 µL F30, 2 μL F30, 2 μL Ligase buffer, 7 μL ddH2O, 1 μL Ligase
- F44: 8.1 μL F44, 1.9 μL F30, 2 μL Ligase buffer, 7 μL ddH2O, 1 μL Ligase
- Ligation was incubated overnight at 16 °C
Digestion of GSY5 (Streptactin; F27)
Investigator: LK
Aim of the experiment: Restricting GSY5 into StrepActin fragment (Streptavidin mutant) and Leptin fragment (Leptin is for Volker)
Procedure:
- Restriction mix:
32µl DNA (F27)
10µl ddH2O
1µl of each enzyme (SpeI, HindIII, SapI)
5µl cut smart buffer
Next Steps:
- Preperative gel, 1,5% Agarose, cut out and purify bands at 80 Bp and 425 Bp. (80 is leptin for volker and 425 is StreptActin for further cloning into pASK)
Results:
Tuesday, June 21st
Plating of BioBricks that didn't survive sequencing
Investigator: JB
Aim of the experiment: Instead of directly inoculating cultures with the transformed cells from the bacterial agar that was sent by the HQ, the bacterial agar was now used for overnight plating on agar plates containing the appropriate antibiotic resistance.
Procedure: Two methods were used for each BioBrick: For the first method, an inoculation loop was used for plating directly. For the second methods, the inoculation loop was used to inoculate a 50 µl LB culture that
Sequencing of various plasmids
Investigator: CG
Aim of the experiment: Sequencing of various plasmids with either VF2 or VR
Procedure: Refer to the “Inventarliste” for detailed informations on sequencing codes and primers. Sequencing batches were prepared according to manufacturer's protocol (15 µL plasmid DNA (50-100 µM) and 2 µL sequencing primer)
PCR of gene synthesises (iGEM_1) (mRuby_EGFR)
Investigator: CG
Aim of the experiment: iGEM_1_mRuby_EGFR (F28) for preparing the EGFR signal (at about 100 bp)
Procedure: Approach:
- 1µl DNA (diluted, 1:10)
- 25 µl 2x MasterMix
- 0,5µl dNTPs
- 2,5 µl forward Primer VF2
- 2,5µl reverse Primer VR
- Fill up with water up to 50 µl
Setup: (calculated with NEB calc. http://tmcalculator.neb.com/#!/)
- 98°C_1min initiation step
- 98°C_10sec
- 66°C_30sec > step 2-4: 35 repeats
- 72°C_30sec
- 72°C_2min final step
- 4°_hold
Transformation of Ligations of F50 (StrepTag), F51 (EspP) and F52 (mRuby3) in pSB1C3
Investigator: JH
Aim of the experiment: Transformation of StrepTag (F50), EspP (F51) and mRuby3 (F52) in XL1 blue
Procedure: transformation in competent E. Coli XL1 blue according to protocol + rescue (repeat due to incubation with LB+Cam ! )
Result: plates (LB Cam) in incubator for further processing (37 °C)
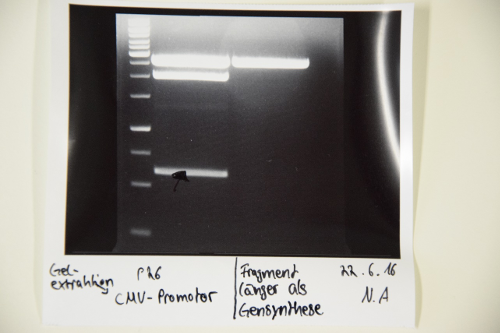
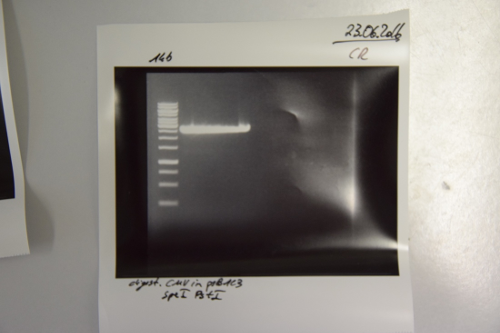
Digestion of P9 (pASK75), P74 (pSB1C3), P26 (CMV promotor) and F47 (BirA)
Investigator: JB
Aim of the experiment: Digestion of P9 and P74 to gain the vectors pASK75 and pSB1C3, respectively, as well as digestion of F47 (PCR fragment of BirA) and P26 (CMV promoter) for the ultimate creation of biobricks
Procedure: P74 was digested in a total volume of 30 µl, while the others were digested in a total volume of 50 µl. Enzymes used were:
- P74: XbaI and PstI
- P9: HindIII and XbaI
- F47: Xba I and PstI (didn’t work)
- P26: SpeI and PstI
The digestions were incubated at 37°C for 40 minutes and were thereafter inactivated at 80°C for 5 mins.
Inoculation of cultures for minipreps for P76 (pCat + strong RBS)
Investigator: JB
Procedure: Inoculation of 2x5 ml cultures with P76 clones. Incubation at 37°C overnight.
Wednesday, June 22nd
Minipreps of P79 (pCat+RBS)
Investigator: JB
Procedure: According to the manufacturer's protocol.
Results: Concentration 212 ng/µl. Sent to sequencing
Transformation of ligation F56 (pASK75(Streptactin)), P60 (pSB1C3 (pC+AraC+pBad+GFPc)) , P70 (pSB1C3 (short linker))
Investigator: CG
Aim of the experiment:' Transformation of pASK75-Streptactin (F56) into E. coli XL1 blue, K577881 (P60) into BL21 and K243004 (P70) in XL1
Procedure: transformation in competent E. Coli XL1 blue according to protocol
Result: plates (LB + appropriate antibiotic) in incubator for further processing (37 °C)
Inoculation of colonies from K157001 (EGFR signal peptide), EspP (F50), mRuby (F51), Strep-Tag (F52)
Investigator: CG
Procedure:
- 2x 5 ml LB+Cam (Amp for K157001) media
- Each culture was inoculated with one colony, for ligations 2 colonies each
- Incubation at 37°C overnight
Plateing of K243004 (short linker), K243005 (middle linker) from stab cultures
Investigator: CG
Procedure: For both biobricks: stabing into the agar with the inocluation loop and resuspending in 50 µl LB media. Plating on Amp-agar.
Digestion of P64 (EGFR sgnal peptide) and P71 (CD4 signal peptide) for cloning into F57 (pSB1C3 (CMV promotor))
Investigator: CG
Aim of the experiment: Digestion of P67 and P71 for cloning the resulting fragments into the digested F57 fragement.
Procedure: both plasmids were digested in 50 µl, 3 µg for each plasmid DNA. 1 µl of XbaI and PstI, 5 µl Buffer, 20 µl DNA, 23 µl H2O. The digestions were incubated at 37°C overnight.
Preparative agarose gels for separation of digestion of P64 (EGFR sgnal peptide) and P71 (CD4 signal peptide)
Investigators: NA, JB
Aim of the experiment: Separation of digestion fragments of both digested PCR fragments as well as vectors
Procedure:
- P74: XbaI and PstI -> Fragment corresponding to the pSB1C3 vector isolated
- P9: HindIII and XbaI -> Fragment corresponding to the pASK75 vector isolated
- F47: Xba I and PstI (didn’t work) –> only one fragment at 3 kbp present (weird since that was a gene synthesis pcr)
- P26: SpeI and PstI -> Fragment corresponding to the cmv-promoter isolated
- Gelextraction of P9, P74 and P26 was executed by manufacturers protocol (Qiagen)
Results:
- F55: pASK75 (2,9 ng/µl)
- F56: pSB1C3 (23,6 ng/µl)
- F57: CMV-Promotor (5,9 ng/µl)
PCR of GSY2 (IRES-BirA) with O17/18
Investigators: LK
Aim of the experiment: Amplification of BirA, using new dilution of GSY2 this time, because last 2 PCRs showed confusing results (bands at 2500)
Procedure:
- PCR was performed according SOP
- Primers: O17 and O18
- Annealing at 68°C
Next Steps:
- PCR-Purification
- Analytical Gel
- Ligation into pSB1C3
QuickChange-PCR of pASK75 with O21 and O22
Investigators: LK
Aim of the experiment: Deleting NdeI from pASK (P03)
Procedure:
- QC-PCR was performed according to SOP
- Primers: O-021 and O-022
- Annealing at 68°C
- Elongation time: 3:30 min
Next Steps:
- Digestion with DpnI at 37°C for 1h
- Purification?
- Preperative Gel (band at 960), purification
- Ligation into pSB1C3
Verification of PCR and Digestion of F28 (gene synthesis 1)
Investigator: NA, LK
Aim of the experiment: Verification of successful PCR via gel electrophoresis and preparation of EGFR
Procedure:
- Gel electrophoresis with 1 kb-ladder
- Digestion: 42µl of PCR Purification, 1,5 µl of each enzyme (NgoMIV and SpeI), 5µl of cut smart buffer.
- Incubation at 37°C over night
Results:
Next steps:
- Preparative gel and gel extraction
- Ligation into pSB1C3
Thursday, June 23th
Miniprep of E. coli Xl1-Blue transformed with ligation product P80/81 (mRuby3 K1/2), P82/83 (EspP K1/2), P84/85 (StrepTag K1/2) and Trafo of K157001
Investigator: Jan, Julian
Aim of the experiment: Miniprep of E. coli Xl1-Blue transformed with ligation product F50(K1,2), F51(K1,2), F52(K1,2) and Trafo of K157001
Procedure:
- Miniprep was performed after manufacturer's protocol (QIAprep Miniprep, QIAGEN)
- Concentrations:
| Plasmid | c [ng/µl] |
| P80 | 432,7 |
| P81 | 294,8 |
| P82 | 450,5 |
| P83 | 479,0 |
| P84 | 108,0 |
| P85 | 356,0 |
| P86 | 47,2 |
PCR of gene synthesises
Investigator: CR
Aim of the experiment: iGEM_1_mRuby_EGFR (F28) for preparing the EGFR signal (at about 100 bp)
Procedure: Approach:
- 1µl DNA (diluted, 1:10)
- 25 µl 2x MasterMix
- 0,5µl dNTPs
- 2,5 µl forward Primer VF2
- 2,5µl reverse Primer VR
- Fill up with water up to 50 µl
Setup: (calculated with NEB calc. http://tmcalculator.neb.com/#!/)
- 98°C_1min initiation step
- 98°C_10sec
- 66°C_30sec > step 2-4: 35 repeats
- 72°C_30sec
- 72°C_2min final step
- 4°_hold
QuickChange-PCR of pASK with O21 and O22
Investigators: CR
Aim of the experiment: Digestion of PCR product with DpnI to digest methylated DNA
Procedure:
PCR purification of BirA and mRuby/EGFR
Investigator: CR
Aim of the experiment: purification of PCR
Procedure: PCR purification via the purification kit according to the manufacturer's protocol The samples were renamed:
- BirA: F59
- mRuby/EGFP: F60
The samples were analysed on a 1% agarose gel. Samples were prepared after SOP.
Results:
Transformation of P60 (K577881; pC+AraC+pBad+GFPc K1) and QC P3 (pASK75(SA1))
Investigator: EF, JB
Aim of the experiment: Transformation of Q CP3 in XL1 blue; transformation of p60 in BL21
Procedure: transformation in competent E. Coli BL21 and XL1blue according to protocol
1µl p60 in BL21 7 µl Q CP3 in XL1 blue
Result: plates (+ rescue plates) incubating
Cloning of CD4- and EGFR-signal peptide into pSB1C3-CMV
Investigator: CR
Aim of the experiment: Digestion of P71 and P69 Procedure:
- Digestions were prepared as following:
- 20 µl CD4 (P71); 1 µl XbaI; 1 µl PstI; 5 µl CutSmart; 23 µl ddH2O
- 7 µl pSB1C3-CMV; P26; 1 µl PstI; 1 µl SpeI; 2 µl CutSmart; µl ddH2O
- 20 µl EGFR-signal peptide (P67);1 µl XbaI; 1 µl PstI; 5 µl CutSmart; 23 µl ddH2O
- Digestions were incubated for 3 h at 37 °C
- The Samples CD4- and EGFR-signal peptide were applied to a 2% and the pSB1C3-CMV to a 1% agarose Gel
Results:
--> passt
--> passt
Next steps:
- Ligation of
Cloning of EGFR (F62) / CD4 (F61) signale peptide into pSB1C3-CMV (F63)
Investigator: CG
Aim of the experiment: Gelextraction and ligation of EGFR- and CD4-signal peptide into pSB1C3 and transformation
Procedure:
- Gel extraction according to manufacturers protocol (Qiagen): F61 (CD4) 2 ng/µl, F62 (EGFR) 0.6 ng/ µl, F63 (CMV): 45 ng/ µl
- Ligation: 1 µl T4 Ligase, 2 µl Ligase buffer, 7 µl H20, 1.8 µl vector and 8.2 µl EGFR insert or 4.3 µl vector and 5.7 µl CD4 insert
- Transformation according to SOP
- Plating on LB-Cam agar
Friday, June 24th
Analytical digestion and gelelectrophoresis of P83 (EspP K2) and P83 (StrepTag K2)
Investigator: Julian, Niklas, Luisa
Aim of the experiment: Analytical digestion and gelelectrophoresis of P82/83 (EspP K1/2) and P84/85 (StrepTag K1/2).
Procedure:
- Batch for analytical digestion for P82-P85 with EcoRI-HF
| volume | reagent |
| 0.5/1.0 µl | Plasmid DNA (-/P84) |
| 1 µl | CutSmart buffer (10x) |
| 0.5 µl | EcoRI-HF(10 U/µl) |
| 8/7.5 µl | ddH2O (-/P84) |
| =10 µl | TOTAL |
Sequencing of P80( mRuby3 K1), P83 (EspP K2) and P85 (StrepTag K2)
Investigator: Julian
Aim of the experiment: Sequencing of P80(mRuby3 K1), P83 (EspP K2) and P85 (StrepTag K2)
Procedure:
Sequencing batch were prepared after manufacturer's protocol. (15 µl of plasmid DNA (50 - 100 ng) and 2 µl sequencing primer). Sequencing primer VF2 was used
The different vectors we sequenced received the following barcodes:
- mRuby3 in pSB1C3 (P80): FR11326590
- EspP in pSB1C3 (P83): FR11326588
- Streptag in pSB1C3 (P85): FR11326587
Transformation of E. coli XL1 blue with F64 (quickchanged P3(pSAm1))
Investigator: Niklas
Aim of the experiment: Transformation of E. coli XL1 blue.
Procedure:
- CaCl2 competent E. coli XL1-Blue cells were put out from the stock in -80 °C freezer and were gently thawed on ice.
- 1 µl of DNA was added to 50 µl of competent cells and gently mixed.
- 30 min incubation on ice
- 5 min. heat shock at 37 °C
- Adding of 950 µl LB-medium to each tube.
- The cell suspension was plated on ampicillin plates (inclusive rescue plate) and incubated over night at 37 °C.
Gelextraction of F67(BirA), F68(mRuby), F69(EGFR-TMD), F70(pSB1C3) and F71(pSB1C3)
Investigator: Niklas
Aim of the experiment: Gelextraction of F67(BirA(Digest. F59 [EcoRI; SpeI])), F68(mRuby(Digest. F60 [NgoMIV; SpeI])), F69(EGFR-TMD(Digest. F60 [NgoMIV; SpeI]), F70(pSB1C3(digest. P74 [NgoMIV; SpeI]) and F71(pSB1C3(digest. P74 [EcoRI; SpeI])
Procedure:
Gelextraction was performed by manufacturers protocol (Qiagen).
Cloning of F59 (BirA) and F60 (EGFR-TMD) into pSB1C3
Investigator: CR
Aim of the experiment: Generate biobricks with EGFR-TMD and BirA
Procedure:
- Digestions were prepared as following:
- 1. xµl F59 (BirA); 1 µl EcoRI; 1 µl SpeI; 3 µl CutSmart; 23 µl ddH2O
- 2. 2 µl P74 (pSB1C3 (RFP-generator); 1 µl NgoMIV; 1 µl SpeI; 3 µl CutSmart; 23 µl ddH2O
- 3. xµl F60 (mRuby/EGFR); 1 µl NgoMIV; 1 µl SpeI; 3 µl CutSmart; 23 µl ddH2O
- 4. 2 µl P74 (pSB1C3 (RFP-generator), 1 µl EcoRI; 1 µl SpeI; 3 µl CutSmart; 23 µl ddH2O
- Digestion was incubated for 3 h at 37 °C
To Do:
- Gel (already prepared)
- Gelextraction
- Ligation
- Trafo
Results:
1. band: P74(NgoMiV, SpeI) 2. band: P74 (EcoRI, SpeI)
1. band: F59 2. band: F60
Transformation of EGFR-TMD-pSB1C3 and BirA-pSB1C3 ligations
Investigator: JB
Procedure: According to protocol. Plates were incubated overnight at 37°C.
Chemical biotinylation of BSA
Investigator: MT, JB
Procedure: BSA was chemically biotinylated with a 20x, 40x and 60x molar excess in two different buffers:100 mM Bicin (pH 8.85) and 100 mM borate (pH 8.85) with 50 mM NaCl respectively.
-> A total of six different tubes.
After one hour incubation at RT, the samples were put in the freezer
Inoculation of liquid cultures (F65 and F66)
Investigator: JB
Procedure:
- Clones were picked for inoculation of 5 ml liquid cultures containing the appropriate antibiotic
- Incubation overnight at 37°C/shaking
Saturday, June 25th
Miniprep of E. coli Xl1-Blue transformed with P60 (mRuby/EGFR),F58 (Ligation pASK75 + Streptactin), F65 (CMV + CD4), F66 (CMV + EGFR), P70 (Short Linker)
Investigator: Niklas
Procedure:
- Miniprep was performed after manufacturer's protocol (QIAprep Miniprep, QIAGEN)
- Concentrations:
| Plasmid | c [ng/µl] |
| P87 | 81 |
| P88 | 34,5 |
| P89 | 86,3 |
| P90 | 108,5 |
| P91 | 417,4 |
Analytical digestion and gelelectrophoresis of F64 (quickchanged P3, pASK75(SA1)) for verification of success of quickchange
Investigator: Niklas
Procedure:
- Analytical digestion with NdeI and gelelectrophoresis. If quickchange worked there should be a band at about 3200 bp (only one restriction site left)
- Incubation over night at room temperature.
- Analytical gelelectrophoresis was performed at 90 V for 60 min.
Results:
Just a band showing a few bp (Primer), there is no plasmid band -> Quickchange did not work
Inoculation of colonies from Ligation of F69 + F70 (EGFR-TMD in pSB1C3) and F44 + F33 (mRuby in pSB1C3)
Investigator: Niklas
Procedure:
- 6x 4 ml LB+Cam media
- Each culture was inoculated with one colony
- Incubation at 37°C overnight
Sunday, June 26th
Miniprep of E. coli Xl1-Blue transformed with ligation product F44 + F30 (mRuby3 in pSB1C3), F66 + F70 (CMV+EGFR in pSB1C3)
Investigator: Luisa
Aim of the experiment: Extracting F44 + F30 (mRuby3 in pSB1C3), F66 + F70 (CMV+EGFR in pSB1C3) from E.coli XL-1-blue
Procedure:
- Miniprep was performed after manufacturer's protocol (QIAprep Miniprep, QIAGEN)
- Concentrations:
| Plasmid | c [ng/µl] |
| P92 | 162,9 |
| P93 | 447,9 |
Repetition of Quick-Change PCR of P3 (pASK + SAm1)
Investigator: Luisa
Procedure:
- The QC-PCR was performed according the SOP.
- Reaction Mix:
| volume | reagent |
| 1,25 µl | Primer O21 |
| 1,25 µl | Primer O22 |
| 1 µl | dNTP-mix |
| 5 µl | Pfu-Ultra-II reaction buffer |
| 1 µl | template DNA (1:10 dilution of p3) |
| 0,5 µl | Pfu-Ultra-II Polymerase |
| 40,5 µl | ddH2O |
- digestion of PCR-Product with DpnI for 1h at 37°C
- now labeled P94
Transformation of E. coli XL1 blue with P94 (quickchanged P3(pSAm1))
Investigator: Luisa
Aim of the experiment: Transformation of E. coli XL1 blue.
Procedure:
- CaCl2 competent E. coli XL1-Blue cells were put out from the stock in -80 °C freezer and were gently thawed on ice.
- 10 µl of DNA was added to 100 µl of competent cells and gently mixed.
- 30 min incubation on ice
- 5 min. heat shock at 37 °C
- Adding of 950 µl LB-medium to each tube.
- The cell suspension was plated on ampicillin plates (inclusive rescue plate) for pASK (F72) and on chloramphenicol plates for P92 and P93 and incubated over night at 37 °C in the incubator.
Analytical digestion and gelelectrophoresis of P92 (mRuby), P93 (CMV + EGFR) and P94 (pASK75)
Investigator: Luisa
Aim of the experiment: Analytical digestion and gelelectrophoresis of P92 (mRuby), P93 (CMV + EGFR) and P94 (pASK75).
Procedure:
- Batch for analytical digestion for P92 and P93 with EcoRI-HF and PstI-HF
| volume | reagent |
| 1 µl | Plasmid DNA |
| 1 µl | CutSmart buffer (10x) |
| 0.5 µl | EcoRI-HF(10 U/µl) and PstI-HF (10 U/µl) for P92/93, NdeI (10 U/µl) for P94 |
| 7.5/7 µl | ddH2O |
| =10 µl | TOTAL |
Results:
- 1. band (P92): no mRuby at 700bp visible, only empty vector
- 2. band (P93): empty vector and EGFR --> perfect
- 3. band (P94): no signal at all --> repetition of QC-PCR
Week 7 (June 27th - July 3rd)
Monday, June 27th
Sequencing of P67 (EGFR-Signalpeptid)
Investigator: Niklas
Procedure:
Sequencing batch was prepared after manufacturer's protocol. (15 µl of plasmid DNA (100 ng) and 2 µl sequencing primer (VF2))
FR11326586
Repetition of Quick-Change PCR of P3 (pASK + SAm1)
Investigator: Luisa
Procedure:
- The QC-PCR was performed according the SOP.
- Reaction Mix:
| volume | reagent |
| 1,25 µl | Primer O21 |
| 1,25 µl | Primer O22 |
| 1 µl | dNTP-mix |
| 5 µl | Pfu-Ultra-II reaction buffer |
| 1 µl | template DNA (1:10 dilution of p3) |
| 0,5 µl | Pfu-Ultra-II Polymerase |
| 40,5 µl | ddH2O |
- Digestion of PCR-Product with DpnI for 1h at 37°C.
- Transformation of 10µl into component E.coli XL-1-blue, according to SOP (1h incubation at 37°C necessary despite AmpR).
PCR of Genesynthesis 3 and 4
Investigator: Luisa
Aim of Experiment: Amplification of Genesynthesis 3 (contains BAP and IGKappa) and 4 (contains A3C5-tag and BM40)
Procedure:
- The PCR was performed according the SOP.
- Reaction Mix:
| volume | reagent |
| 2,5 µl | Primer VF2 |
| 2,5 µl | Primer VR2 |
| 1 µl | dNTP-mix |
| 10 µl | Q5 Polymerase reaction buffer |
| 1 µl | template DNA (1:10 dilution of p3) |
| 0,5 µl | Q5-Polymerase |
| 18 µl | ddH2O |
- Setup: iGEM_standard (Promega-cycler)
| temperature | time |
| 98°C | 2min |
| 98°C | 10sec |
| 66°C | 30sec |
| 72°C | 30sec |
| 72°C | 2min |
| 4°C | hold |
- the batches were then purified using the Quiagen PCR-Purification Kit.
Analytical digestion and gelelectrophoresis of P88 (pASK75(Steptactin)), P89 (pSB1C3(CMV+CD4) and P90 (pSB1C3(CMV+EGFR-Signal peptide)
Investigator: Niklas
Aim of experiment: Analytical digestion and gelelectrophoresis of P88 (pASK75 + Streptactin, former F58), P89 (CMV + CD4, former F65) and P90 (CMV + EGFR-signal-peptide, former F66)
Procedure:
- Batches for analytical digestions:
P88: EcoRI
P89: EcoRI and PstI
P90: EcoRI
| volume | reagent |
| 5,8/2,3/1,8 µl | Plasmid DNA (P88/P89/P90) |
| 1 µl | CutSmart buffer (10x) |
| 0.5 µl | EcoRI-HF(10 U/µl)/ PstI |
| required amount for total volume of 10 µl | ddH2O |
Ligation of F67 (BirA) and F71 (vector fragment pSB1C3), Transformation of E. coli XL1 blue afterwards
Investigator: Niklas
Aim of the experiment: Ligation of F67 (BirA) and F71 (empty pSB1C3), Transformation of E. coli XL1 blue afterwards.
Procedure:
| volume | reagent |
| 2,4 µl | Vektor |
| 7,6 µl | Insert |
| 2 µl | 10X DNA-Ligase-buffer |
| 1 µl | T4-Ligase |
| 7 µl | ddH2O |
| =20 µl | TOTAL |
- CaCl2 competent E. coli XL1-Blue cells were taken out of stock in -80 °C freezer and were gently thawed on ice.
- 7 µl of DNA was added to 100 µl of competent cells and gently mixed.
- 30 min incubation on ice
- 5 min. heat shock at 37 °C
- Adding of 750 µl LB-medium to each tube.
- Incubation for 1 hour at 37 °C
- The cell suspension was plated on Cam-plates (inclusive rescue plate) and incubated over night at 37 °C in the cell-culture shaker.
- next step: analytic digestion of transformation was successful
Digestion of PCR on genesynthesis 3 and 4, and pSB1C3
Investigator: Luisa
Aim of experiment: Division of Leptin, IGKappa, A3C5, BM40 and BAP using SapI, HindIII, XbaI, AgeI for both batches.
Procedure:
- Batches for analytical digestions:
| volume | reagent |
| 1 µl each | enzyme (SapI, HindIII, XbaI, AgeI) |
| 5 µl | CutSmart buffer (10x) |
| 41 µl | DNA (purified PCR-products of GSY3 and 4) |
- Additionally 10µg of the vector P74 was digested with XbaI and AgeI in 100µl batch (2µl of each enzyme, 10µl of Cut-Smart buffer). Digestion was performed over night and purified via gelelectrophoresis and gelextraction according to the manufacturer's protocoll. --> Now labeled F80.
Analytical digestion and gelelectrophoresis of P80 , P78 and P85
Investigator: Julian
Aim of experiment: Analytical digestion and gelelectrophoresis of P80 (mRuby3), P78 (NanoLuc) and P85 (Strep-Tag)
Procedure:
- Batches for analytical digestions:
- P80 and P78: EcoRI and AgeI
- P85: EcoRI and NgoMIV
| volume | reagent |
| 10 µl | Plasmid DNA |
| 32 µl | ddH2O |
| 5 µl | CutSmart buffer (10x) |
| 1.5 µl | each enzyme(10 U/µl)/ PstI |
| 50 µl | TOTAL |
Ligation of F75 (mRuby) and F76 (NanoLuc) into F74 (pSB1C3 with Strep-Tag) and Transformation into E. coli XL1 blue
Investigator: Luisa
Aim of the experiment: Ligation of F75 (mRuby) and F76 (NanoLuc) into F74 (pSB1C3+Strep-Tag), Transformation of E. coli XL1 blue afterwards.
Procedure:
| volume | reagent |
| 4,3µl(for F75), 8,2µl (for F76) | Vector |
| 12,7µl (F75), 8,8 (F76) | Insert |
| 2 µl | 10X DNA-Ligase-buffer |
| 1 µl | T4-Ligase |
| =20 µl | TOTAL |
- Ligation was incubated at RT for 1,5h.
- CaCl2 competent E. coli XL1-Blue cells were taken out of stock in -80 °C freezer and were gently thawed on ice.
- 7 µl of DNA was added to 100 µl of competent cells and gently mixed.
- 15 min incubation on ice
- 5 min. heat shock at 37 °C
- Adding of 950 µl LB-medium to each tube.
- Incubation for 1 hour at 37 °C
- The cell suspension was plated on Cam-plates (inclusive rescue plate) and incubated over night at 37 °C in the incubator.
Chemical biotinylation of BSA
Investigator: Niklas
Procedure:
- BSA was chemically biotinylated with a 20x and 40x molar excess:
- 10 ml of 100 mM borate buffer with 50 mM NaCl (pH 8.85)
- dissolve BSA (10 mg/ml)
- Add biotin-NHS-ester: 20,5 mg for 40x molar excess
- reaction over night
Tuesday, June 28th
Preparative digestion and gelelectrophoresis of P83 (EspP) and P29 (DoubleTerminator)
Investigator: Julian
Aim of experiment: Preparative digestion and gelelectrophoresis of P83 (EspP) and P29 (DoubleTerminator)
Procedure:
- Batches for analytical digestions:
- P83: EcoRI and SpeI
- P29: EcoRI and XbaI
| volume | reagent |
| 6.5/13 µl | Plasmid DNA (P83/P29) |
| 35.5/29 µl | ddH2O (P83/P29) |
| 5 µl | CutSmart buffer (10x) |
| 1.5 µl | each enzyme |
| 50 µl | TOTAL |
- Preparative gelelectrophoresis was performed at 80 V for 80 min
Gelelectrophoresis of F73 (pSB1C3 digst. EcoRI, AgeI)
Investigator: Julian
Aim of experiment: gelelectrophoresis of P80 (mRuby3), P78 (NanoLuc) and P85 (Strep-Tag)
Procedure: Preparative gelelectrophoresis was performed at 100 V for 45 min using a pocket spanning over the full width of the 1% agarose gel
Wednesday, June 29th
Transformation of F83 (EspP-Fragment in Double Terminator + pSB1C3), F84 (GSY3 + pSB1C3) F85 (GSY4 + pSB1C3)
Investigator: Niklas
Procedure:
- CaCl2 competent E. coli XL1-Blue cells were put out from the stock in -80 °C freezer and were gently thawed on ice.
- 7 µl of DNA was added to 50 µl of competent cells and gently mixed.
- 30 min incubation on ice
- 5 min. heat shock at 37 °C
- Adding of 950 µl LB-medium to each tube.
- The cell suspensions were plated on Cam-plates (inclusive rescue plate) and incubated over night at 37 °C.
Thursday, June 30th
Transformation of new E. coli XL1-blue (P75), additional plating of untransformated XL1-blue
Aim of experiment: Quality assurance
Investigator: Niklas
Procedure:
- the new competent E. coli XL1-Blue cells were put out from the stock in -80 °C freezer and were gently thawed on ice.
- 1 µl of DNA was added to 50 µl of competent cells and gently mixed.
- 30 min incubation on ice
- 5 min. heat shock at 37 °C
- Adding of 950 µl LB-medium to each tube.
- The cell suspension was plated on Cam-plates (inclusive rescue plate) and incubated over night at 37 °C.
- additional plating of untransformated XL1-blue on Tet-, Cam-, Amp- and Kan-plates
Result:
- After 12 hous of incubation there were some (~20) colonies on the 50µl-plate transformed with Cam-resistence-plasmids. The transformation has been succesful, though cells grow slow.
- There were no colonies on the kan-, amp- and cam- plates with the untransformed E.Coli. Hence we can assume that there are no other plasmids with resistences against kan, amp or cam in our batch of competent cells.
There are cells on the tet-plate. This was expected.
Friday, June 31th
Transformation of P9 (pASK with Streptavidin WT) and QC-PCR product into new E. coli XL1-blue
Aim of experiment: Tranforming pASK without NdeI-cutting point into E.coli (two batches from diffrent approches were tested). And Transforming pASK_SA1 into E.coli XL-1-blue.
Investigator: Luisa
Procedure:
- the new competent E. coli XL1-Blue cells were put out from the stock in -80 °C freezer and were gently thawed on ice.
- 10 µl of DNA was added to 50 µl of competent cells and gently mixed.
- 15 min incubation on ice
- 30 sec. heat shock at 42 °C
- Adding of 950 µl LB-medium to each tube. 1h hour incubation at 37°C.
- The cells were plated out on Amp-plates (inclusive rescue plate) and incubated over the weekend at 30 °C.
Week 8 (July 04–10)
Monday, July 04
Repetition of the Transformation of QC-PCR product into new E. coli XL1-blue
Aim of experiment: Tranforming pASK without NdeI-cutting point into E.coli (two batches from diffrent approches were tested).
Investigator: Luisa
Procedure:
- the new competent E. coli XL1-Blue cells were put out from the stock in -80 °C freezer and were gently thawed on ice.
- 10 µl of DNA was added to 50 µl of competent cells and gently mixed.
- 15 min incubation on ice
- 30 sec. heat shock at 42 °C
- Adding of 950 µl LB-medium to each tube. 1h hour incubation at 37°C.
- The cells were plated out on Amp-plates (inclusive rescue plate) and incubated over the weekend at 30 °C.
Transformation of P9 (Streptavidin in pASK75) into E. coli BL-21
Aim of experiment: Tranforming pASK with Streptavidin into E.coli BL-21 for Expression.
Investigator: Niklas
Procedure:
- the new competent E. coli BL-21 cells were put out from the stock in -80 °C freezer and were gently thawed on ice.
- 1 µl of DNA was added to 50 µl of competent cells and gently mixed.
- 15 min incubation on ice
- 5 min. heat shock at 37 °C
- Adding of 950 µl LB-medium to each tube.
- The cells were plated out on Amp-plates (inclusive rescue plate) and incubated over the weekend at 30 °C
Transformation of F94 (hGH polyadenylation sequence and Nanoluc with StrepTag)
Investigator: Javier
Procedure:
- CaCl2 competent E. coli XL1-Blue cells were put out from the stock in -80 °C freezer and were gently thawed on ice.
- 7 µl of DNA was added to 50 µl of competent cells and gently mixed.
- 30 min incubation on ice
- 45 sec. heat shock at 42 °C
- Adding of 950 µl LB-medium.
- The cell suspensions were plated on Cam-plates (inclusive rescue plate) and incubated over night at 37 °C.
Miniprep of E. coli Xl1-Blue transformed with ligation product P108 and P113 (EspP-Fragment in Double Terminator, F81+F82), P109 and P110 (GSY3+ F73 vector AgeI+XbaI ), P111 and P112 (GSY4+ F73 vector AgeI+XbaI)
Investigator: Javier
Aim of the experiment: Extracting P108 and P113 (EspP-Fragment in Double Terminator, F81+F82), P109 and P110 (GSY3+ F73 vector AgeI+XbaI ), P111 and P112 (GSY4+ F73 vector AgeI+XbaI) from E.coli XL-1-blue
Procedure:
- Miniprep was performed after manufacturer's protocol (QIAprep Miniprep, QIAGEN)
- Concentrations:
| Plasmid | c [ng/µl] |
| P108 | 116,2 |
| P109 | 86,6 |
| P110 | 136,8 |
| P111 | 144,8 |
| P112 | 163,2 |
| P113 | 123,3 |
Preparative digestion and gelelectrophoresis of P74 (RFP-Gen.)
Investigator: Julian
Aim of experiment: Preparative digestion and gelelectrophoresis of P74 (RFP-Gen.)
Procedure:
- Batch for analytical digestion:
- P74: EcoRI and SpeI
| volume | reagent |
| 2.5 µl | Plasmid DNA |
| 39.5 µl | ddH2O |
| 5 µl | CutSmart buffer (10x) |
| 1.5 µl | each enzyme |
| 50 µl | TOTAL |
- Preparative gelelectrophoresis was performed at 90 V for 45 min
Result: pSB1C3 (EcoRI und SpeI) F95
Cloning of F96 (BirA in pSB1C3)
Investigator: Julian
Procedure:
- CaCl2 competent E. coli XL1-Blue cells were put out from the stock in -80 °C freezer and were gently thawed on ice.
- 10 µl of DNA was added to 50 µl of competent cells and gently mixed.
- 5 min incubation on ice
- 30 s heat shock at 37 °C
- Adding of 950 µl LB-medium
- Incubation at 37 °C
- The cell suspension was plated on Cam-plates (inclusive rescue plate) and incubated over night at 37 °C
Tuesday, July 05th
Preparing of 200 ml TB medium for inoculation of 2 L medium on Thursday
Investigator: Niklas
Procedure:
- 2.4 g Tryptone 4.8 g yeast extract
- 800 µl Glycerine
- adjust to 180 ml with ELGA-water
- seperately: 3.29 g H2KPO4 + 0,46 g K2HPO4 with ELGA water (Volume = 20 ml)
Digestion of F49 (BirA) with EcoRI and SpeI and cloning in F95 (pSB1C3)
Investigator: Clemens
Procedure:
- Digestion of F49 with EcoRI and SpeI
| volume | reagent |
| 20 µl | Plasmid DNA (F49) |
| 5 µl | CutSmart buffer (10x) |
| 1 µl | EcoRI-HF(10 U/µl) |
| 1 µl | SpeI(10 U/µl) |
| 23 µl | ddH2O |
| =50 µl | TOTAL |
- Digestion was incubated for 1.5 h at 37°C
- Digestion was applied to a 1% agarose-gel
- Gel ran at 80V for 35 min
File:Muc16 digestF49 0507.jpeg
- BirA had a length of 1012 bp
- Upper probe was cut from the gel
- BirA was purified with gelextraction which was performed by manufacturer's protocol
- Concentration was measured
- [F97]= 12.4
- Ligation of F97 into pSB1C3 (F95)
| volume | reagent |
| 1.2 µl | Vector: pSB1C3 (F95) |
| 8.8 µl | Insert: BirA (F97) |
| 2 µl | T4 DNA Ligase buffer |
| fill up to 20 µl | dd H2O |
| 1 µl | T4 ligase |
| =21 µl | TOTAL |
- Ligation was incubated at RT for 30 min
- Transformation:
- Transformation was performed after SOP
- 7 µl were used for transformation in E. coli XL1blue
Sequencing of P108 - P112
Aim of experiment: Sequencing
Investigator: Niklas
Procedure:
- sequencing batches after protocol with following barcodes:
- P108 (EspP-Fragment in Double Terminator (F83)) FR11326572 (VF2)
- P109 (GSY3+ F73 Leervektor AgeI+XbaI (F84)) FR11326576 (VF2)
- P110 (GSY3+ F73 Leervektor AgeI+XbaI (F84)) FR11326575 (VF2)
- P111 (GSY4+ F73 Leervektor AgeI+XbaI (F84)) FR11326574 (VF2)
- P112 (GSY4+ F73 Leervektor AgeI+XbaI (F84)) FR11326573 (VF2)
NEB Turbo CaCl2-competent cells
Investigator: Vivien
Procedure:
- Inoculate 50 mL LB with 0.5 mL overnight culture of NEB Turbo cells
- Incubate at 37 °C for 7 h; final OD500: 0.357
- Very little growth in the course of the last hour; OD500 0.45 required
- Experiment discontinued. Inoculated 3 further colonies in 5 mL LB for o/n growth (37 °C). Repeat experiment on Wed, July 6
Miniprep P114 (NanoLuc + StrepTag + Poly-A in pSB1C3(F94))
Investigator: Niklas
Procedure:
- Miniprep was performed after manufacturer's protocol (QIAprep Miniprep, QIAGEN)
Analytical digestion and gelelectrophoresis of P114 and P115
Investigator: Niklas
Aim of the experiment:
- Analytical digestion and gelelectrophoresis of P114 and P115 (NanoLuc + StrepTag + Poly-A in pSB1C3 K1 and K2)
Procedure:
- Batch for analytical digestion for P114 and P115 with EcoRI+PstI
| volume | reagent |
| 1.75 µl | Plasmid DNA P114/P115 |
| 1 µl | cut smart (10x) |
| 0.5 µl | EcoRI |
| 0.5 µl | PstI |
| 6,25 µl | ddH2O |
| =10 µl | TOTAL |
- Incubation for 45 min at 37 °C.
- Analytical gelelectrophoresis was performed at 90 V for 60 min.
Results:
File:Muc16 digestP114 P115 0507.jpeg
| 1 kbp ladder DNA ladder | P114 | P115 |
| X | X |
Inoculation of Quick change of P88 (pASK75 (Streptactin)) preculture
Investigator: Clemens
Procedure:
- four colonies were picked from plate
- Cells were inoculated in 5 mL LB medium (ampicillin 1:1000)
Analytical digestion of P108 and P113 (both EspP + double terminator (F83)) with EoRI and PstI
Investigator: Clemens
Procedure:
- Digestion of F49 with EcoRI and SpeI
| volume | reagent |
| 1 µl | Plasmid DNA (P108/P113) |
| 1 µl | CutSmart buffer (10x) |
| 0.5 µl | EcoRI-HF(10 U/µl) |
| 0.5 µl | PstI(10 U/µl) |
| 7 µl | ddH2O |
| =10 µl | TOTAL |
- Digestion was incubated for 1.5 h at 37°C
- Digestion was applied to a 1% agarose-gel
- Gel ran at 80V for 35 min
File:Muc16 analyt.digest.P108 P113 0507.jpeg
- P108 and P113 showed the right bands
Wednesday, July 06th
Sequencing of P108 (EspP-Fragment in Double Terminator (F83))
Investigator: >Niklas
Procedure:
- sequencing batches after protocol with following barcodes:
- P108 (EspP-Fragment in Double Terminator (F83)) FR11326572 (VR)
Digestion of P75 (RFP-Generator) with HincII + dephosphorylation
Investigator: Niklas
Aim of experiment: Blunt-Ligation of BirA into pSB1C3
Procedure:
- Digestion of P75 (RFP-Generator) with HincII + dephosphorylation
| volume | reagent |
| 3 µl | Plasmid DNA (P75) |
| 2 µl | CutSmart buffer (10x) |
| 0.5 µl | HincII |
| 1 µl | Fast AP (for dephosphorylation) |
| 14.5 µl | ddH2O |
| =20 µl | TOTAL |
- Digestion was incubated for over night at 37°C
- next steps: PCR of GSY2 with O17 and O18 + phosphorylation, Ligation in digested pSB1C3 afterwards
Cloning of A3C5 Tag into the MiddleLinker-Plasmid
Investigator: Christoph
Aim of experiment: Cloning of A3C5 Tag into the MiddleLinker-Plasmid
Procedure:
- Digestion of Middle_Linker Plasmid (P69) with AgeI and PstI
| volume | reagent |
| 0.3 µl | Plasmid DNA (69) |
| 2 µl | CutSmart buffer (10x) |
| 1 µl | AgeI |
| 1 µl | PstI |
| 1 µl | Fast AP (for dephosphorylation) |
| 14.5 µl | ddH2O |
| =20 µl | TOTAL |
| volume | reagent |
| 0.3 µl | Plasmid DNA (P112) |
| 2 µl | CutSmart buffer (10x) |
| 1 µl | NgomIV |
| 1 µl | PstI |
| 14.5 µl | ddH2O |
| =20 µl | TOTAL |
- Digestion was incubated for over night at 37°C, heat inactivation at 80°C for 20 min.
| volume | reagent |
| 2 µl | digested Middle-Linker (F99) |
| 8 µl | digested A3C5 (F100) |
| 2 µl | 10x Ligase Buffer |
| 1 µl | Ligase |
| 7 µl | ddH2O |
| =20 µl | TOTAL |
- Transformation according to SOP in E. coli XL1 blue
Picking of of E. coli XL1 blue with Streptactin in quickchanged pASK75, F84 (digested GSY3), F85 (digested GSY4)
Investigator: Niklas
Procedure:
- Streptactin in quickchanged pASK75: Colonies were picked from Amp-plates. (10x)
- F84/F85: Colonies were picked from Cam-plates. (8x each)
- Picked pipette tips was transferred into cell-culture tubes with air-permeable, sterile cover. Each tube contains 4 mL of LB-medium + 4 µL of the proper antibiotic.
- These tubes were transferred in a cell culture shaker at 37 °C and were incubated overnight
Quick Change of mRuby Plasmids
Investigator: Christoph
Aim of experiment: Correction of wrong amino acid within the mRuby sequence
Procedure:
QC according to manufacturers protocol
| volume | reagent |
| 10 ng (= 0,3 µl 1:10) | Plasmid DNA (80) |
| 5 µl | 10x Pfu Ultra II Buffer |
| 1.25 µl | O23 |
| 1.25 µl | O24 |
| 0.5 µl | Pfu Ultra II |
| 1 µl | dNTPs |
| 40.7 µl | ddH2O |
| =50 µl | TOTAL |
| volume | reagent |
| 10 ng (= 0,3 µl 1:10) | Plasmid DNA (100) |
| 5 µl | 10x Pfu Ultra II Buffer |
| 1.25 µl | O23 |
| 1.25 µl | O24 |
| 0.5 µl | Pfu Ultra II |
| 1 µl | dNTPs |
| 40.7 µl | ddH2O |
| =50 µl | TOTAL |
- PCR: 2 min. 98°C initial denaturation, 10 sec. 98°C, 30 sec. Annealing st 60 °C, 5 min 68°C, 35 Cycles
- 1 h at 37°C DpnI Digestion
- Transformation according to SOP in E. coli XL1 blue, QC on mRuby-Strep (F103) was also transformed in E. coli Turbo cells for further investigating the growth rate
Miniprep of Quick Change of pASK75-Streptactin + analytical digestion with Nde1
Investigator: Jan
Procedure:
- Miniprep using the kit according to the manufacturer's protocol.
- Although cultures seemed to have grown properly, very low and unreliable DNA concentrations were obtained via Nanodrop measurement and no fragment at all could be detected via analytical agarose gel electrophoresis after 90 minutes digestion with Nde1
Creation of competent NEB Turbo cells
Investigator: Jan
Procedure:
- 250 µl of precultures were used to inoculate 50 ml LB-medium
- After an OD=0.45 was reached, cells were put on ice and made competent according to the manufacturer's protocol
- 50 µl aliquots of competent cells were frozen in liquid nitrogen and stored at -80°C until further use
Inoculation of a BL21 pBAD-GFP (P60) preculture
Investigator: Jan
Procedure:
- A single clone was picked in order to inoculate 5 ml LB medium (+Cam)
- Culture was grown overnight at 37°C
Inoculation of a BL21 preculture for Streptavidin production (P8)
Investigator: Jan
Procedure:
- A clone was picked for the inoculation of 5 ml LB medium
- After 6-8 hours of incubation, the 5 ml preculture was used for the inoculation of a 50 ml LB preculture
Thursday, July 07
PCR Amplification of birA (GSY 2)
Investigator: Vivien
Aim: Amplify birA gene
Procedure:
- Prepared PCR mix (25 μL reaction volume): NEB Q5 High-Fidelity 2× Master Mix (12.5 μL), ddH2O (9.5 μL), F29 (1 μL), O17 and O18 (1 μL each)
- Cycling protocol: start 1.5 min, 98 °C; 35 cycles 30 s, 98 °C – 45 s, 72 °C – 30 s, 72 °C; final elongation 5 min, 72 °C; end 4 °C
- Purified via Quiagen PCR Purification kit following manufacturer's instructions. DNA eluted from column by adding 50 μL EB buffer and heating at 37 °C for 5 min prior to centrifugation.
Results: DNA concentration: 82.7 ng/μL. Stored as F102 at –20 °C
Induction of Streptavidin
Investigator: Vivien
Aim: Induce streptavidin production
Procedure:
- Inoculated 2 L TB medium with 50 mL o/n pre-culture and added 2 mL ampicillin (10 mg/mL in 50% EtOH)
- Let grow (37 °C, 200 rpm) until OD500=0.622 (c. 2.5 h)
- Induced with anhydrotetracycline (1:10,000 i.e. 200 μL of 2 mg/mL stock)
- Let grow (37 °C, 200 rpm) for 4 h
Results: Induced culture for harvesting (see below)
Production of eGFP
Investigator: Clemens, Vivien, Christoph
Aim: Produce eGFP
Procedure:
- Inoculated 50 mL LB+Cam medium with 0.5 mL o/n pre-culture
- Let grow (37 °C, 200 rpm) until OD500=0.58
- Induced with 0.1 mM arabinose (i.e. 100 μL of 50 mM stock)
- After 7 h of induction the cells were stored in the fridge overnight for maturation of the GFP-chromophore
Results:
Harvesting of Streptavidin
Investigator: Niklas
Aim of the experiment: Recombinant expression and purification of streptavidin
Procedure:
- After expression, cultures were transferred into centrifuge tubes and spun down in the centrifuge (4 °C, 5000 rpm, 20 min, F4X1L rotor)
- The supernatant was discarded, and the pellet was transferred into a Falcon and frozen
- next steps: resuspending and homogenizing in the PANDA
Friday, July 08
Analytical Digest of mRuby3 Constructs
Aim of experiment: Verify integration of mRuby3 constructs in F103 and F104
Investigator: Vivien
Procedure:
- Mixed 16 μL F103 (or 8 μL F104 plus 8 μL ddH2O) with 2 μL 10× CutSmart buffer and 1 μL each of EcoRI-HF and PstI-HF (20 μL total volume)
- Incubated for 45 min at 37 °C
- Subjected to gel electrophoresis (1% agarose, 80 V, 120 min)
Results: No band around 700 bp as expected for mRuby3 constructs
Picking clones and inoculating cultures for F101 and F103
Investigator: Jan
Procedure:
- All clones available (only two for F101, one for F103) were picked and used to inoculate 5 ml of LB medium containing the appropriate antibiotic
- Cultures were incubated at 37°C overnight
Solubilization, lysis and unfolding of streptavidin
Investigator: Jan
Procedure:
- According to protocol -> lysis of pellet (19.2 g) in 58 ml lysis buffer (50 mM Tris/HCl pH 8, 1 mM EDTA)
- As only two liters of medium were used, the Panda couldn't be utilized for cell lysis -> French Press was used
- Cells were additionally submitted to ultrasonic treatment
- Centrifugation of cells, casting away the supernatant and resolubilizing the pellet in 5 ml GdmCl (6M, pH 1.5)
Sequencing of P88
Aim of experiment: Verification of QC
Investigator: Julian
Procedure:
Sequencing batch was prepared after manufacturer's protocol. (15 µl of plasmid DNA (100 ng) and 2 µl sequencing primer (PR1))
FR11326565
Transformation of P75 (RFP-Generator), F101 (middle linker+ A3C5), P125 (dig. GSY4) into E. coli XL1-blue and P75 in NEB Turbo E. coli
Investigator: Niklas, Vivien
Procedure:
- the competent E. coli cells were put out from the stock in -80 °C freezer and were gently thawed on ice.
- 1 µl of DNA (6 µl for Ligation F101) was added to 50 µl of competent cells and gently mixed.
- 30 min incubation on ice
- 5 min. heat shock at 37 °C
- 30 min incubation on ice
- Adding of 950 µl LB-medium to each tube.
- The cells were plated out on Cam-plates/Amp-plate for F101 (inclusive rescue plate) and incubated over the weekend at 30 °C
QuickChange-PCR of P80 (O23 and O24) and P100 (O19 and O20)
Investigators: Niklas
Aim of the experiment: Changing of amino acid
Procedure:
- the batches were split, each batch contained only one primer, the batches were pooled after 15 cycles (1 µl Polymerase is added)
- Annealing at 55°C
| volume | reagent |
| 1.25µl | O23/O24 and O19/O20 |
| 0,5 µl | dNTP-mix |
| 1 µl | 1:100 P80 / P100 |
| 2.5 µl | Pfu Ultra II-buffer |
| 0.5 µl | Pfu Ultra II Polymerase |
| 19.25 µl | dd H2O |
| 4 x 25 µl | TOTAL |
Preperative gelelektrophoresis of F102 (PCR of BirA) + phosphorylation and ligation into pSB1C3
Investigator: Luisa
Aim of experiment: Blunt-Ligation of BirA into pSB1C3
Procedure:
- The whole PCR-product was applied to a 1% agorose gel. After runnig the gel two lines where visible: one at 1000Bp and one at ~500Bp.
- The 1000 Bp band was cut out and purified with the Gelextraction kit according to manufacturer's protocoll. Concentration: 41,5ng/µl.
- The DNA was phosphorylated as follows:
| volume | reagent |
| 15 µl | DNA (~1pmol) |
| 2 µl | Ligase buffer (10x) |
| 1 µl | T4 Polynukleotide-Kinase (for phosphorylation) |
| 2 µl | ddH2O |
| =20 µl | TOTAL |
- The batch was incubated for 20 min at 37°C and then the enzyme was inactivated for 10 min at 75°C.
- The phosphorylated DNA was then ligated into prepared (HincII-digested) pSB1C3 as follows:
| volume | reagent |
| 2,3 µl | Vector |
| 7,7 µl | Insert (BirA) |
| 2 µl | Ligase Buffer (10x) |
| 1 µl | T4 Ligase |
| 7 µl | ddH2O |
| =20 µl | TOTAL |
- next steps: Transformation into E.coli Xl-1-blue.
Digestion of P78, P123 (for BAP-Nanoluc-construct),P122 (IgKappa for CMV-IgKappa-construct), P89, P114 (for CMV-EGFR-Nanoluc-StrepTag-PolyA-construct)
Investigator: Luisa
Aim of experiment: Getting of BAP-Nanoluc-construct, CMV-IgKappa-construct and CMV-EGFR-Nanoluc-StrepTag-PolyA-construct.
Procedure:
- DNA was digested according to the following table:
| volume | reagent |
| 3 µg (for P78, P123 and P122) and 1,5 µg (for P89, P114) | DNA |
| 5 µl | CutSmart buffer (10x) |
| 1,5 µl of NgoMIV and PstI | for P78 |
| 1,5 µl of AgeI and PstI | for P123 |
| 1,5 µl of XbaI and PstI | for P122 |
| 1,5 µl of AgeI and PstI | for P189 |
| 1,5 µl of NgoMIV and PstI | for P123 |
| up to 50µl | ddH2O |
| =50 µl | TOTAL |
Results: Concentrations: P78 now F106: 2,7 ng/µl, P89 now F107: 14,4 ng/µl. Gelextractions of P114, P122 and P123 didn't contain DNA. (To few DNA available)
- next steps: Retransformation of verified clones (P114, P89) into Xl-1-blue for amplification of plasmid.
Repetition of digestion of P122 and P123 with more DNA (10µg!), then Gel with 100Bp ladder!
Repetition of digestion of P123 (BAP for BAP-Nanoluc-construct) and P122 (IgKappa for CMV-IgKappa-construct)
Investigator: Luisa
Aim of experiment: Getting of BAP-Nanoluc-construct, CMV-IgKappa-construct
Procedure:
- DNA was digested according to the following table:
| volume | reagent |
| 10 µg --> 20µl | DNA |
| 5 µl | CutSmart buffer (10x) |
| 1,5 µl of AgeI and PstI | for P123 |
| 1,5 µl of XbaI and PstI | for P122 |
| up to 50µl | ddH2O |
| =50 µl | TOTAL |
- Incubation at 37°C over night.
- next steps: Gel with 100Bp ladder and Gelex. Then Ligation with prepared vectors from former digestion (F106 an F63)
Week 9, July 11–17
Monday, July 11
MiniPreps of pSb1C3-Middle Linker+A3C5, -BM40, -CMV-EGFR Retrafo, Streptactin in QC pASK75
Investigator: Christoph
Aim of the experiment: Plasmid preparation, analytic digestion, sequencing
Procedure:
- MiniPrep was performed according to manufacturer's protocol (QIAprep MiniPrep, Qiagen)
- Analytic digestion with 0.25 µL EcoRI, 0.25 µL PstI (HF) for pSB1C3 plasmids and 0.5 µl NdeI for pASK75-Streptactin, 1 µL SmartCut Buffer, 3 µL plasmid DNA, 5.5 µL H2O
- 5 µL on 1% agarose gel electrophoresis
- plasmid assignment was tricky because of confusion during the weekend Minipreps
Results:
- pASK75-Streptactin clone 1 and 2 were not visible
- verified Plasmid names are available after sequencing
- Lane 1: 5 µl 1 kb Ladder
- Lane 2: 5 µL Plasmid F101 K1 (seems to be pSB1C3-RFP-Generator)
- Lane 3: 5 µL Plasmid F101 K2 (seems not to be pSB1C3-RFP-Generator)
- Lane 4: 5 µl 100 bp Ladder
- Lane 5: 5 µL P125 K1 (seems not to be pSB1C3-RFP-Generator)
- Lane 6: 5 µL P125 K2 (seems to be pSB1C3-RFP-Generator)
Preperative digestion of NanoLuc + StrepTag + Poly-A (F112) and cloning behind CMV-EGFR (F107)
Investigator: Christoph, Niklas
Aim of the experiment: preperative digestion for cloning of the construct behind of CMV-EGFR
Procedure:
- 15 µl P115 (NanoLuc + StrepTag + Poly-A), 1 µl NgoMiV, 1 µl PstI, 3 µl CutSmart buffer, 10 µl H2O
- Incubation for 2 h at 37°C
- 1% gel electrophoresis
Photo
- gel extraction according to SOP
- Ligation of 2 µl F107 (AgeI, PstI) + 8 µl F112 (NgomIV, PstI) with 2 µl Ligase buffer, 7 µl ddH2O, 1 µl T4-Ligase for 40 minutes
- CaCl2-competent E. coli XL1-Blue cells were taken from the stock in –80 °C freezer and gently thawed on ice
- 7 µl of DNA was added to 100 µl of competent cells and gently mixed
- 30 min incubation on ice
- 5 min heat shock at 37 °C
- Added 1 ml LB medium to each tube
- Incubation for 55 min at 37 °C in the 180 rpm cell-culture shaker
- 60 µl of the cell suspension was plated on one chloramphenicol plate
- The rest were centrifuged for 1 min at 13,000 rpm and the supernatant was dicarded
- The pellet was resuspended in 60 µl of LB-medium and this concentrated cell suspension was plated again on a new chlorampenicol plate (=rescue plate).
Cloning of Nanoluc behind BAP
Investigator: Christoph, Niklas
Aim of the experiment: Cloning of Nanoluc behind BAP based (testing fast cloning strategy)
Procedure:
- 5 µl P78 (Nanoluc), 0.5 µl NgoMiV, 0.5 µl PstI, 1 µl CutSmart buffer, 2.5 µl H2O, 0.5 SacI = F108
- 4 µl P123 (pSB1C3-BAP), 0.5 µl AgeI, 0.5 µl PstI, 1 µl CutSmart buffer, 3 µl H2O, 1 µl FastAP = F109
- Incubation for 2 h at 37°C, 15 min heat inactivation
- Ligation: 2 µl F108 (Nanoluc) + 8 µl F109 (BAP), 2 µl buffer, 1 µl Ligase, 7 µl H2O
- Transformation:
- CaCl2-competent E. coli XL1-Blue cells were taken from the stock in –80 °C freezer and gently thawed on ice
- 7 µl of DNA was added to 100 µl of competent cells and gently mixed
- 30 min incubation on ice
- 5 min heat shock at 37 °C
- Added 1 ml LB medium to each tube
- Incubation for 55 min at 37 °C in the 180 rpm cell-culture shaker
- 60 µl of the cell suspension was plated on one chloramphenicol plate
- The rest were centrifuged for 1 min at 13,000 rpm and the supernatant was dicarded
- The pellet was resuspended in 60 µl of LB-medium and this concentrated cell suspension was plated again on a new chlorampenicol plate (=rescue plate).
Gel extraction of IgKappa (F111) and cloning behind CMV in pSB1C3 (F63)
Investigator: Christoph, Niklas
Aim of the experiment: gel extraction after digestion for cloning of the construct behind of CMV
Procedure:
- 1% gel electrophoresis
Photo
- gel extraction according to SOP
- Ligation of 6.1 µl F111 ( XbaI, PstI) + 3.9 µl F63 (SpeI, PstI) with 2 µl Ligase buffer, 7 µl ddH2O, 1 µl T4-Ligase for 40 minutes
- CaCl2-competent E. coli XL1-Blue cells were taken from the stock in –80 °C freezer and gently thawed on ice
- 7 µl of DNA was added to 100 µl of competent cells and gently mixed
- 30 min incubation on ice
- 5 min heat shock at 37 °C
- Added 1 ml LB medium to each tube
- Incubation for 55 min at 37 °C in the 180 rpm cell-culture shaker
- 60 µl of the cell suspension was plated on one chloramphenicol plate
- The rest were centrifuged for 1 min at 13,000 rpm and the supernatant was dicarded
- The pellet was resuspended in 60 µl of LB-medium and this concentrated cell suspension was plated again on a new chlorampenicol plate (=rescue plate).
Retrafo of P123 (digest. GSY3-BAP)
Investigator: Niklas
Procedure:
- CaCl2-competent E. coli XL1-Blue cells were taken from the stock in –80 °C freezer and gently thawed on ice
- 1 µl of DNA was added to 100 µl of competent cells and gently mixed
- 30 min incubation on ice
- 5 min heat shock at 37 °C
- Added 1 ml LB medium to each tube
- Incubation for 55 min at 37 °C in the 180 rpm cell-culture shaker
- 60 µl of the cell suspension was plated on one chloramphenicol plate
- The rest were centrifuged for 1 min at 13,000 rpm and the supernatant was dicarded
- The pellet was resuspended in 60 µl of LB-medium and this concentrated cell suspension was plated again on a new chlorampenicol plate (=rescue plate).
Digestion of quickchanged mRuby (without StrepTag) and transformation of E. coli XL1-Blue afterwards
Investigator: Niklas
Procedure:
- the PCR-product is digested by 1 µl DpnI (cuts methylated DNA)
- CaCl2-competent E. coli XL1-Blue cells were taken from the stock in –80 °C freezer and gently thawed on ice
- 7 µl of DNA was added to 100 µl of competent cells and gently mixed
- 30 min incubation on ice
- 5 min heat shock at 37 °C
- Added 1 ml LB medium to each tube
- Incubation for 55 min at 37 °C in the 180 rpm cell-culture shaker
- 60 µl of the cell suspension was plated on one chloramphenicol plate
- The rest were centrifuged for 1 min at 13,000 rpm and the supernatant was dicarded
- The pellet was resuspended in 60 µl of LB-medium and this concentrated cell suspension was plated again on a new chlorampenicol plate (=rescue plate).
- next steps: 4 Minipreps, test digestion, Sequencing
QuickChange-PCR of P100 (O23 and O23)
Investigators: Julian, Niklas
Aim of the experiment: Changing of amino acid in mRuby3
Procedure:
- the batches were split, each batch contained only one primer, the batches were pooled after 15 cycles (1 µl Polymerase is added)
- Annealing at 55°C
| volume | reagent |
| 1.25µl | O23/O24 |
| 0,5 µl | dNTP-mix |
| 1 µl | 1:100 P100 |
| 2.5 µl | Pfu Ultra II-buffer |
| 0.5 µl | Pfu Ultra II Polymerase |
| 19.25 µl | dd H2O |
| 4 x 25 µl | TOTAL |
Repeated digestion of P75 (RFP-Generator) with HincII + dephosphorylation
Investigator: Julian
Aim of experiment: Blunt-Ligation of BirA into pSB1C3
Procedure:
- Digestion of P75 (RFP-Generator) with HincII + dephosphorylation
| volume | reagent |
| 6 µl | Plasmid DNA (P75) |
| 2 µl | CutSmart buffer (10x) |
| 1 µl | HincII |
| 1 µl | Fast AP (for dephosphorylation) |
| 11 µl | ddH2O |
| =20 µl | TOTAL |
- Digestion was incubated for 2 hours at 37°C
Ligation of phosphorylated F102 (PCR of BirA) into F113 (RFP-Generator P75 digest. HincII)
Investigator: Julian
Aim of experiment: Blunt-Ligation of BirA into pSB1C3
Procedure:
- The phosphorylated DNA (prepared on July 8th) was ligated into F113 (HincII-digested RFP-Generator) as follows:
| volume | reagent |
| 4,5 µl | Vector F113 |
| 5,7 µl | Insert (BirA) |
| 2 µl | Ligase Buffer (10x) |
| 1 µl | T4 Ligase |
| 7 µl | ddH2O |
| =20 µl | TOTAL |
- next steps: Transformation into E.coli NEB Turbo.
Tuesday, July 12
Transformation of BirA–RFP generator ligation (F114) and Re-Transformation of P114
Investigator: Vivien
Aim: Transform NEB Turbo cells
Procedure:
- Followed standard transformation protocol (SOP) using 1 μL and 10 μL of P114 and F114, respectively
- Incubated at 37 °C for x h
- Plated on LB Cam (50 μL + rescue)
Week 10, July 18–24
Week 11, July 25th - July 31st
Week 12, August 1st - August 7th