Introduction
Biosafety is defined as "the discipline addressing the safe handling and containment of infectious microorganisms and hazardous biological materials"[1] According to the U.S. Department of Health and Human Services and the CDC. For our team, the security and safety measures of our project have had the highest priority. Since there are many inherent risks of our work in the iGEM laboratory, we have decided to thoroughly present the dangers that we have analyzed while working at it. Firstly it is relevant to mention that we used non-pathogenic E. coli strains like BL21 and Xl1-blue for the cloning part of the project, which are typical strains used in the labs. Nevertheless, we have had an extreme caution to prevent any spreading of used living organisms, since this were S1 genetically modified organisms. The field of synthetic biology provides us with the possibility to work with living organisms and modify them. There are many safety measures that are very important to consider while investigating and working at the laboratory for this purpose. Since the base of synthetic biology is the work with genetically modified organisms (GMO), it is necessary to look out for risk factors like spreading of the organism or infection from the investigators. It is also important to highlight that molecular biology works at the laboratory involve the use of some harmful chemicals, which is another aspect of the safety to be considered.
Safety in the laboratory
The Laboratory in which the iGEM Project has been conducted, at the Chair of biological chemistry is classified as a laboratory with the biosafety level S1. According to the German law of the regulation of GM technology (Gesetz zur Regelung der Gentechnik) there are 4 levels of biosafety, namely from S1 to S4. In the Level S1 there is considered that no risk for human health or the environment is present.[2] In order to guarantee the integrity of all the team members and the environment, we took preventive steps prior to the beginning of our lab work. All team members that were going to be performing wet lab tasks were required to attend a lecture about the risks and precautions while working with GMOs. It was conducted by Dr. Martin Schlapschy in charge of the safety of the laboratory. In this presentation information about the regulation of biology by governmental institutions in Germany and the obligations each laboratory has to fulfill e.g. having a good and ethical practice was also included. After this, all team members were given an introduction to the laboratory, in order to get to know all important safety features as well as localization of the proper lab equipment. Factors like disposal of chemicals, GMOs and handling of dangerous chemicals or sensitive cells (cell culture) were specifically emphasized and thoroughly explained.
It is important to underline some important safety measures that have to be taken while conducting molecular biology experiments:
- The use of Ethidium bromide, since it is considered to be a mutagenic chemical compound. The use of gloves and safety glasses was mandatory while handling it. [3]
a-rna-purification- analysis/nucleic-acid-gel-electrophoresis/dna-stains/ etbr.html This measures also applied for compounds like Hydrochloric acid and Ammonia, additionally this were to be handled with the protection of a fume hood, due to the volatility of some of them.
- The use of a special protective mask in order to protect the eyes from the UV Light while analyzing gels.
- The use of certain machines at the laboratory posed some risks as well. As an example the use of big centrifuges, which if mishandled could cause harm to the investigators.
Biosafety: No danger to scientists, the population or the environment
In the daily work with genetically modified microorganisms and cells, it is always important to consider various aspects of biosafety. When planning and conducting experiments one has to evaluate all potential risks and dangerous posed to human health and the environment thereby and take all eventual necessary precautionary measures (precaution principle). Under this principle, general contact with the biological material, for instance, shall be reduced to a minimum. As our project relies on a variety of wet lab work all constructs and experiments were carefully checked ahead of execution. Our work in the laboratory was determined not to pose any kind of unusual risks neither to the experimenters nor the general public or the environment. The strict containment of the experiments within the lab was ensured at all times. All strains and lines organisms and cells used were specifically designed for lab applications and are therefore not able to survive outside laboratory conditions as no aspect of our project work overrides these restraints.
Biosecurity: No potential misuse or dual-use character in our project
In order to fully ensure that a project does not allow for any harm to be dealt it is also imperative for the scientists to assess possible harmful misuse of an invention. Relevant to such an analysis are whether the genetic construct can be used directly or indirectly to arm microorganisms. Constructs for example that allow for the production of any kind of toxins or dangerous chemicals as well as genetic code that allows for the integration or transmission of DNA.
As we have evaluated our project accordingly we were not able to find any aspect of our work which could be used or modified to serve exterior unwanted purposes. No part allows for the production of any kind of harmful substances nor the spread of the construct themselves.
The debate surrounding bioprinting
The topic of bioprinting, due to the fact of being relatively young, is still currently being discussed in international ethics and safety forums and in courts. This is why we consider it to be essential to briefly present and discuss the main concerns around bioprinting. It is important to mention that there is a broad spectrum of perspectives and disciplines, in which key questions about tissue printing can be analyzed.
The world is facing a lot of challenges nowadays with the constant improvement and advances in the field of biology. These being questions about the ethic of some projects and furthermore the regulation of the reproduction of the same experiments, in the case of its success. This is why it is very important to consider all these political, social and ethical issues bonded to the scope and effect of the project. It is worth mentioning how there is a big debate about organ transplantation already since it first became possible in 1945 when the first kidney was successfully transplanted (HRSA). [4] With bioprinting a lot of new questions around patentability, affordability towards the different social strata, clinical safety and social acceptance arise and are to be discussed and dealt with.
One of the biggest security concerns around the use of 3D printed organs for transplantation is the tort liability of physicians, added to the intellectual property debate of 3D produced organs. Considering the fact that the transplantation of these organs needs still years of research of becoming a reality, the governments and regulation organisms are facing a great challenge in regulating the use and manufacture of 3D printed organs. To illustrate this problem, as an example, the Food and Drug Administration (FDA) is in charge of regulating medical devices as artificial hearts. Meanwhile is Organ Donation regulated by other governmental agreements, like in the case of Illinois, the Anatomical gift act. [5]
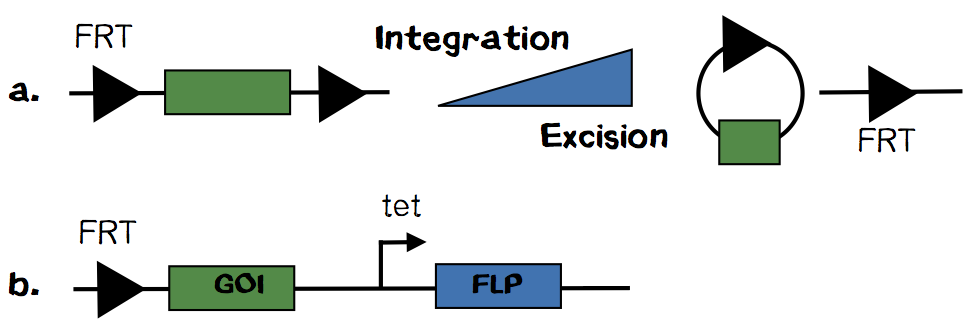
Flp Recombinase - A stable but reversible genetic alteration
In our talks with medical doctors, pharmaceutical companies and the public one question always popped up: „Can you undo the genetic engineering after the synthetic organ is transplanted into a humans body?“
We thought about this tricky question and finally came up with the idea to modify the well established „T-REx“ system. The system is based on a FRT site encoded on a plasmid and another FRT site in the genome. By recombineering of these sites, catalysed by Flp recombinase, the plasmid is integrated. But more likely is the excision of an integrated plasmid. The Flp recombinase is encoded on a plasmid without any episomale replication origin. Therefore it is diltued in every cell divison. That‘s why loss of integrated plasmid gets more unlikely over time. Upon plasmid loss the integration cassette can no longer be excised and the cell is stable genetically altered.
How could we utilize this system for our safety approach? We are suggesting to encode the Flp recombinase, on the same plasmid as the integration cassette, under the control of a tight promoter like the tet-promoter. With this design we could ensure that the integration plasmid and the Flp recombinase are always present in any cell. Upon exposure to a suitable stimulus Flp recombinase will be transcribed and excise the integrated plasmid from our synthetic organ.
Interviews
Interview with a member of the national biosafety panel (ZKBS) Professor Dr. Alfons Gierl
This year we invited Professor Dr. Alfons Gierl, a german scientist in the field of genetics and a member of the [http://www.bvl.bund.de/DE/06_Gentechnik/02_Verbraucher/05_Institutionen_fuer_biologische_Sicherheit/02_ZKBS/gentechnik_zkbs_node.html national biosafety panel (ZKBS)] to discuss safety matters of our project but also talk about the potential and future of synthetic biology and his experience in the field of GMO's. In his opinion, synthetic biology can be a promising field of research, if the main goal is its application in the biomedical area since the demand in this area is very high and has the best chance to be accepted by the non-scientist community. In safety questions the [http://www.bvl.bund.de/DE/06_Gentechnik/02_Verbraucher/05_Institutionen_fuer_biologische_Sicherheit/02_ZKBS/gentechnik_zkbs_node.html ZKBS] plays an important role in Germany as it, for example, paraphrases the law to evaluate what risk group a certain GMO belongs to. As he says the current laws in Germany cover most of the recent developments and he is quite sure that they will cover those made in synthetic biology through the next couple of years as well. Since our project deals with the generations of GMO's that can be printed in 3D tissues and could possibly be implanted into a recipient, we wanted to know what he thinks about the risk of GMO release. According to him, the risk is rather small, since the constructs are integrated stably and the GMO's do not have contact with cells of the germline.
We want to thank Professor Dr. Gierl once again for his support and this highly interesting interview.
Interview with Dr. Nina Köhler, Bavarian Health and Food Safety Authority (LGL)
During our Synthetic Biology project we held an interview with Dr. Nina Köhler from the Bavarian Health and Food Safety Authority (LGL). Dr. Köhler is currently working on a project monitoring the latest national and international developments in synthetic biology and on developing strategies for their analytical surveillance. The topic of the interview were the current regulations of Synthetic Biology in the EU and in Germany. Dr. Köhler referred to the “Zentrale Kommission für die Biologische Sicherheit” (ZKBS) at the Federal Office of Consumer Protection and Food Safety [http://www.bvl.bund.de/DE/Home/homepage_node.html (BVL)]. According to the ZKBS all current Synthetic Biology activities (apart from in vitro gene/genome synthesis and protocell research) are regulated by the German Genetic Engineering Act.
We want to thank Dr. Köhler for taking the time and converse with us about our project.
Interview with Dr. Michael Raghunath of the Zürcher university for applied science
To get a better understanding of how our cells would behave after polymerization we talked to Dr. Michael Raghunath of the zuercher university for applied science (ZHAW). Since his research spans the fields of matrix biology, pathochemistry and wound repair he was the perfect fit for us. After explaining our main project and the idea behind it, we talked about possible problems of our polymerization approach. We talked about possible hypoxia, if the polymer reaches a certain diameter and ideas how that could be prevented. He advised us that a higher structural integrity of our polymer would be helpful for the viability of our transfected adherent cells and that the viability could be tested by monitoring the expression of fibronectin and type-4-collagen, as they are part of the extracellular matrix and of cell-cell adhesion.
Since the interview had such a high information load and so many valuable suggestions, it is a shame that we did not have the chance to talk to Dr. Raghunath earlier during the project. His expertise would have been very helpful. Nonetheless we are very thankful that we had the opportunity to talk to Dr. Raghunath and want to thank him for taking the time to discuss about our project with us.
Interview with Dr. Nadine Nottrodt of the Fraunhofer Institute for Laser Technology (ILT)
Dr. Nadine Nottrodt was the project manager of [http://www.artivasc.eu/ ArtiVasc 3D], a project funded by the European Commission, which dealt with the generation of vascularized tissue structures that can be 3D printed by a photolithographic process and used a scaffold for cells to make up new tissue. We were very happy that she agreed to have this interview with us, for the reason that the [http://www.ilt.fraunhofer.de/ ILT] is also involved in more than one project that deals with the scaffold-free generation of 3D cell cultures. After discussing the main idea of our project Dr. Nottrodt was very keen on learning more about how we exactly polymerize our cells and she said she was impressed by what we already had accomplished by that time. Since the binding of streptavidin and biotin is very strong we discussed the influence of the stiffness of our polymerized structure on the viability and division capability of the cells because in former experiments, with a related approach, adipocytes showed a decreased division capability starting at a certain stiffness of a surrounding matrix. After discussing the possible future of our project we talked about the future of scaffold-free 3D bioprinting in general. In Dr. Nottrodt’s opinion, the scaffold-free printing of tissues will have many advantages, the absence of foreign material inside the body and the resulting prevention of possibly toxic by-products just being two of them.
Again we want to thank Dr. Nadine Nottrodt for taking the time to talk to us. It was a very pleasant interview that helped us to classify our project in the light of the recent developments.
A practical contribution to Biosafety in iGEM projects: The kill-switch
Aim of the new designed Thymidine kinase / ganciclovir system
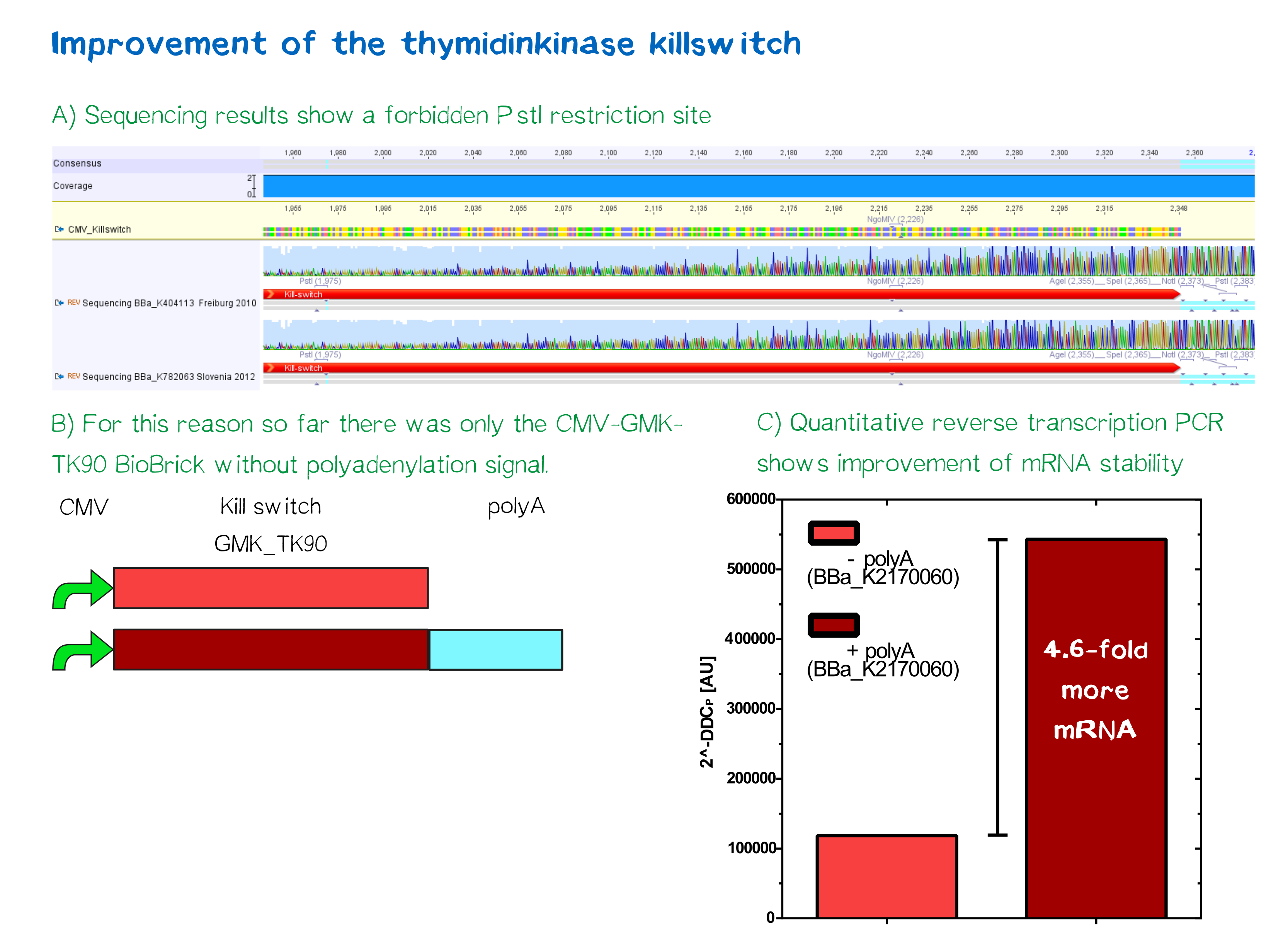
One key factor in guaranteeing the safety of our project and preventing in advance any dangers is the implementation of a well-known kill-switch. In this case, we worked in cooperation with the iGEM-team of Slovenia and Freiburg, who each provided us with their Biobrick ([http://parts.igem.org/Part:BBa_K404113 BBa_K404113] - HSV-thymidine kinase fused to mouse guanylate kinase, iGEM Freiburg 2010 and [http://parts.igem.org/Part:BBa_K782063 BBa_K782063] - CMV-promoter, iGEM Slovenia 2012). This specific kill switch is known as the Thymidine kinase / ganciclovir system. In order to enhance the current Biobrick, we have repaired a point mutation that led otherwise to a pstI restriction location. An additional Poly-A-tail should also stabilize the mRNA later on. For more details, see our experiment protocol further down.
The role of herpes simplex virus thymidine kinase
Thymidine kinase plays an important role in pyrimidine salvage pathway. It catalyzes the monophosphorylation of thymidine to deoxythymidine monophosphate (dTMP) [6]. Human TK is incapable of phosphorylating guanosine analogues as ganciclovir, acyclovir, buciclovir, penciclovir and famciclovir. Since herpes simplex virus TK is able to phosphorylate guanosine analogues, it has been widely tested as a successful suicide gene in combination with ganciclovir, especially for the treatment of cancers and various virus infections, but also as a kill-switch in various iGEM-projects [7].
Freiburg 2010 has first implemented the fusion protein of HSVTK since it conveys higher toxicity results. Plain HSVTK has only limited kinase activity on guanosine nucleosides. To compare GCV to thymidine, it is 500-fold more catalytic active in the case of thymidine than GCV. The specific mutant 30 of HSVTK attributes besides the fusion with mGMK to an even higher improvement in toxicity. For iGEM, it may be also of great interest to take the mouse GMK since it is soluble in E. coli in comparison to its human analogue [8].
After successful transfection of mGMK-TK30 fusion protein the following incubation with one of the mentioned guanosine analogues leads initially to monophosphorylation followed by di- and trimethylation by cellular kinases. The trimethylated state is obviously the active form of the prodrugs [9]. Triphosphorylated guanosine analogs like GCV can be incorporated into nascent DNA and lead to inhibition of DNA replication. This leads to cell death [10].
The Bystander Effect
Toxicity of guanosine analogues on non-targeted cells was also taken into account. The effect of, for instance, GCV on HSV-negative cells seems to be an in vitro result for experiments where cultured cells contained at least 10% HSV-TK-positive cells. Researches address to this phenomenon as the “Bystander Effect”. It is assumed that the bystander effect is based on apoptotic cell death. Apoptotic vesicles of genetically modified cells are known to be phagocytized by nearby, unmodified cells. This could be proved by inhibition of apoptotic vesicle transfer [11].
The reason for selecting HSV-TK in combination with GCV is a result of its high specificity for the guanosine analogue compared to thymidine kinases [12]. At the end, this specificity leads to directed cell death.
In the future case of implanting printed tissues into a patient, the bystander effect supports biosafety. Like it is in the case with tumor cells, we assume that our printed tissue is dividing more frequently than its environment after implanting, especially if the printed tissue evolves malignant. Since a higher frequency of cytokinesis influences transfection positively, printed tissues would be targeted more directly by the bystander effect. Another positive effect is, that the bystander effect needs direct cell-cell contact because a soluble factor seems not to be involved [13]. Although it was demonstrated that phosphorylated GCV metabolites can leak out from cells, only neighboring cells are at risk to be killed by an unknown cellular uptake mechanism independent of gap junctions [14].
Why we use Famciclovir
In order to fulfill the iGEM-competition’s philosophy, we intended to improve current BioBricks and the kill-switch, that was implemented for instance in Freiburg 2010 and Slovenia 2012. Therefore, we did not only erase point mutations in the mGMK-TK30-construct and added a Poly-A-tail, but went on using the current most modern prodrug: famciclovir. First, since for medication 4 common drugs are available on the market, we intended to analyze the differences between them in more detail.
With aciclovir the new era of antiviral therapy started in the 1980s. It is still a widely used drug against Herpes simplex virus type 1 and 2 and Varicella-zoster-virus infections. Ganciclovir is known as the hydroxymethylated form of aciclovir and is known for its better activity against CMV [15]. Replacement of the ether oxygen by a carbon atom reflects the structure of penciclovir. Penciclovir has lower bioavailability than the other two guanosine analogues. Its prodrug famciclovir, with two acetyl groups instead of the hydroxyl groups and without the keto group at the purine (see Figure..). It is well absorbed when orally admitted and rapidly metabolized to penciclovir [16]. Nevertheless, all four guanosine analogues show adverse side effects, especially on the male reproductive system [17].
Metabolism of Famciclovir
The major metabolism of orally admitted famciclovir includes normally a deacetylation followed by oxidation of the purine to form penciclovir. This is how the prodrug works in vivo. To get nearer to in vivo conditions in cell culture assays, the diacetyl ester, famciclovir, should be incubated in extracts of liver, previously processed by aldehyde oxidase and xanthine oxidase or implemented on a cell culture assay with cells that are capable of effectively transforming famciclovir.
To implement a kill switch on famciclovir basis has the clear advantage of using the most actual drug that has higher biocompatibility and bioavailability (41%) in humans. In contrast to some guanosine analogues like aciclovir, the active form of famciclovir, penciclovir, is even phosphorylated much more efficiently. But penciclovir has poor absorbance (1,5%), if it is directly admitted.
Penciclovir is much more efficiently phosphorylated than aciclovir. Nevertheless, in uninfected cells, host cell kinases phosphorylate both guanosine analogues to a small and similar content. Due to its highly preferential metabolism penciclovir is more commonly selected for GMK-TK30 infected cells [18].
Experimental approach
Our Kill-Switch construct consists of a CMV-promoter, the GMK-TK30 and an additional Poly-A-tail. Conceiving the fusion gene GMK-TK30, additional parts were assembled step by step. In order to test the functionality of our Kill-Switch, we transfected HEK293T cells with the construct. 125 mg of Famciclovir were solubilized in 125 ml DMEM (+10% Fetal Calf Serum, +1% Penicillin-Streptomycin). The drug was further diluted in different concentrations ranging from 0 µg/ml, 62,5 µg/ml, 125 µg/ml, 250 µg/ml, 500 µg/ml to 1000 µg/ml. After 96 h incubation cells were stained with DAPI and calcein-AM.
In order to get a clear answer concerning Poly-A-efficiency (or improvement of mRNA stability), we performed an RT-qPCR twice on mRNA isolated of non-transfected, with Poly-A-sequence transfected and without Poly-A-sequence transfected cells, respectively. Each one of the Poly-A/Non-Poly-A cross-point-values was proportioned to the negative control and house-keeping gene values.
Conclusion and discussion
Sequencing results showed a point mutation in both the part by the iGEM team of Freiburg 2010 and the Slovenian part of 2012. The mutation led to a PstI restriction site, that complicated cloning according to iGEM standards. After a site-directed mutagenesis (Quickchange) the restriction site was removed and the sequence hence repaired.
Quantitative analysis by mRNA measuring of Poly-A-sequence addition showed 4.6-fold higher mRNA stability in comparison to the non-Poly-A-construct.
Kill-Switch induction turned out to be difficult when using the prodrug famciclovir. Although HEK293T cells are known to produce aldehyde oxidase [19] and xanthine oxidase [20] the conversion of famciclovir into its active form penciclovir seems not to proceed effectively enough in HEK293T cells in order to be detectable by fluorescence microscopy. Concerning incubation time and drug concentration, we have indeed used up to a 10-fold higher concentration (3110 µM) than for instance Qiu et al. (311 µM) or iGEM-Slovenia 2012 (392 µM) and almost the same as iGEM-Freiburg 2010 (4850 µM). Nevertheless, the assumed effect was almost not observable, which shows that in the case of HEK293T cells famciclovir is not well suited for acting as an inducing drug for the GMK-TK30 Kill-Switch. Therefore, we recommend for in vitro studies to use either ganciclovir or penciclovir, the latter known to be less effective in vitro at least on rat Sertoli cells [21]. Finally, we can only assume that the reason for the weakness of famciclovir as an inducer of apoptosis may be due to its complex metabolism. The advantages of famciclovir lie rather in its high bioavailability after oral administration.
References
- ↑ U.S. Department of Health and Human Services (2009, December). Biosafety in Microbiological and Biomedical Laboratories. Retrieved October 6, 2016, from http://www.cdc.gov/biosafety/publications/bmbl5/BMBL.pdf
- ↑ Bundesministerium der Justiz und für Verbraucherschutz. Gesetz zur Regelung der Gentechnik (Gentechnikgesetz - GenTG)§ 7 Sicherheitsstufen, Sicherheitsmaßnahmen. Retrieved October 06, 2016, from http://www.gesetze-im-internet.de/gentg/__7.html
- ↑ Ethidium Bromide. Retrieved October 06, 2016, from https://www.thermofisher.com/de/de/home/life-science/dn
- ↑ Timeline of Historical Events Significant Milestones in Organ Donation and Transplantation. Retrieved September 03, 2016, from http://www.organdonor.gov/legislation/timeline.html
- ↑ Park, M. H. For a new heart, just click print. Retrieved September 10, 2016, from http://www.globalforumljd.org/cops/legal-and-ethical-aspects-human-organs-3d-bioprinting
- ↑ Kokoris, M. S., & Black, M. E. (2002). Characterization of herpes simplex virus type 1 thymidine kinase mutants engineered for improved ganciclovir or acyclovir activity. Protein science, 11(9), 2267-2272.
- ↑ Moolten, F. L. (1986). Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer research, 46(10), 5276-5281.
- ↑ Ardiani, A., Sanchez-Bonilla, M., & Black, M. E. (2010). Fusion enzymes containing HSV-1 thymidine kinase mutants and guanylate kinase enhance prodrug sensitivity in vitro and in vivo. Cancer gene therapy, 17(2), 86-96.
- ↑ Miller, W. H., & Miller, R. L. (1980). Phosphorylation of acyclovir (acycloguanosine) monophosphate by GMP kinase. Journal of Biological Chemistry, 255(15), 7204-7207.
- ↑ Reardon, J. E. (1989). Herpes simplex virus type 1 and human DNA polymerase interactions with 2'-deoxyguanosine 5'-triphosphate analogues. Kinetics of incorporation into DNA and induction of inhibition. Journal of Biological Chemistry, 264(32), 19039-19044.
- ↑ Freeman, S. M., Abboud, C. N., Whartenby, K. A., Packman, C. H., Koeplin, D. S., Moolten, F. L., & Abraham, G. N. (1993). The “bystander effect”: tumor regression when a fraction of the tumor mass is genetically modified. Cancer research, 53(21), 5274-5283.
- ↑ Moolten, F. L. (1986). Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer research, 46(10), 5276-5281.
- ↑ Freeman, S. M., Abboud, C. N., Whartenby, K. A., Packman, C. H., Koeplin, D. S., Moolten, F. L., & Abraham, G. N. (1993). The “bystander effect”: tumor regression when a fraction of the tumor mass is genetically modified. Cancer research, 53(21), 5274-5283.
- ↑ Drake, R. R., Pitlyk, K., McMasters, R. A., Mercer, K. E., Young, H., & Moyer, M. P. (2000). Connexin-independent ganciclovir-mediated killing conferred on bystander effect-resistant cell lines by a herpes simplex virus-thymidine kinase-expressing colon cell line. Molecular Therapy, 2(5), 515.
- ↑ McGavin, J. K., & Goa, K. L. (2001). Ganciclovir. Drugs, 61(8), 1153-1183.
- ↑ Simpson, D., & Lyseng-Williamson, K. A. (2006). Famciclovir. Drugs, 66(18), 2397-2416.
- ↑ Qiu, R., Horvath, A., & Stahlmann, R. (2016). Effects of four nucleoside analogues used as antiviral agents on rat Sertoli cells (SerW3) in vitro. Archives of Toxicology, 1-7.
- ↑ Hodge, R. V. (1993). Famciclovir and penciclovir. The mode of action of famciclovir including its conversion to penciclovir. Antiviral Chemistry and Chemotherapy, 4(2), 67-84.
- ↑ Moriwaki, Y., Yamamoto, T., Takahashi, S., Tsutsumi, Z., & Hada, T. (2001). Widespread cellular distribution of aldehyde oxidase in human tissues found by immunohistochemistry staining. Histology and histopathology, 16(3), 745-753.
- ↑ Czupryna, J., & Tsourkas, A. (2012). Xanthine oxidase‐generated hydrogen peroxide is a consequence, not a mediator of cell death. FEBS Journal, 279(5), 844-855.
- ↑ Qiu, R., Horvath, A., & Stahlmann, R. (2016). Effects of four nucleoside analogues used as antiviral agents on rat Sertoli cells (SerW3) in vitro. Archives of Toxicology, 1-7.