(→biotINK - a synthetic biology approach to bioprinting) |
(→biotINK - a synthetic biology approach to bioprinting) |
||
| Line 6: | Line 6: | ||
While interactions of biomolecules make up the cellular system, the three-dimensional organization of cells makes up tissues and organs. For decades now, scientists have tried to reconstitute such functional tissues by assembling the cells they are made out of - especially for the field of regenerative medicine, where the acquisition of suitable transplants may mean life or death for the patient. The prospect of creating personalized transplants artificially has since motivated groups all around the world to take part in this endeavour.<ref>Murphy, S. V., & Atala, A. (2014). 3D bioprinting of tissues and organs. Nature biotechnology, 32(8), 773-785.</ref> | While interactions of biomolecules make up the cellular system, the three-dimensional organization of cells makes up tissues and organs. For decades now, scientists have tried to reconstitute such functional tissues by assembling the cells they are made out of - especially for the field of regenerative medicine, where the acquisition of suitable transplants may mean life or death for the patient. The prospect of creating personalized transplants artificially has since motivated groups all around the world to take part in this endeavour.<ref>Murphy, S. V., & Atala, A. (2014). 3D bioprinting of tissues and organs. Nature biotechnology, 32(8), 773-785.</ref> | ||
| − | But not only could bioprinting prove fruitful in the field of personalized medicine - for pharmacological applications, three-dimensional cell culture systems often constitute the first step in testing a potential pharmacological agent. Systems that resemble the human system as much as possible are hereby especially desirable, as they allow a more faithful prediction of a drug's effect ''in vivo'' and may furthermore reduce the need for lab animals.<ref>Griffith, L. G., & Swartz, M. A. (2006). Capturing complex 3D tissue physiology in vitro. Nature reviews Molecular cell biology, 7(3), 211-224.</ref> | + | But not only could [https://2016.igem.org/Team:LMU-TUM_Munich/Demonstrate bioprinting] prove fruitful in the field of personalized medicine - for pharmacological applications, three-dimensional cell culture systems often constitute the first step in testing a potential pharmacological agent. Systems that resemble the human system as much as possible are hereby especially desirable, as they allow a more faithful prediction of a drug's effect ''in vivo'' and may furthermore reduce the need for lab animals.<ref>Griffith, L. G., & Swartz, M. A. (2006). Capturing complex 3D tissue physiology in vitro. Nature reviews Molecular cell biology, 7(3), 211-224.</ref> |
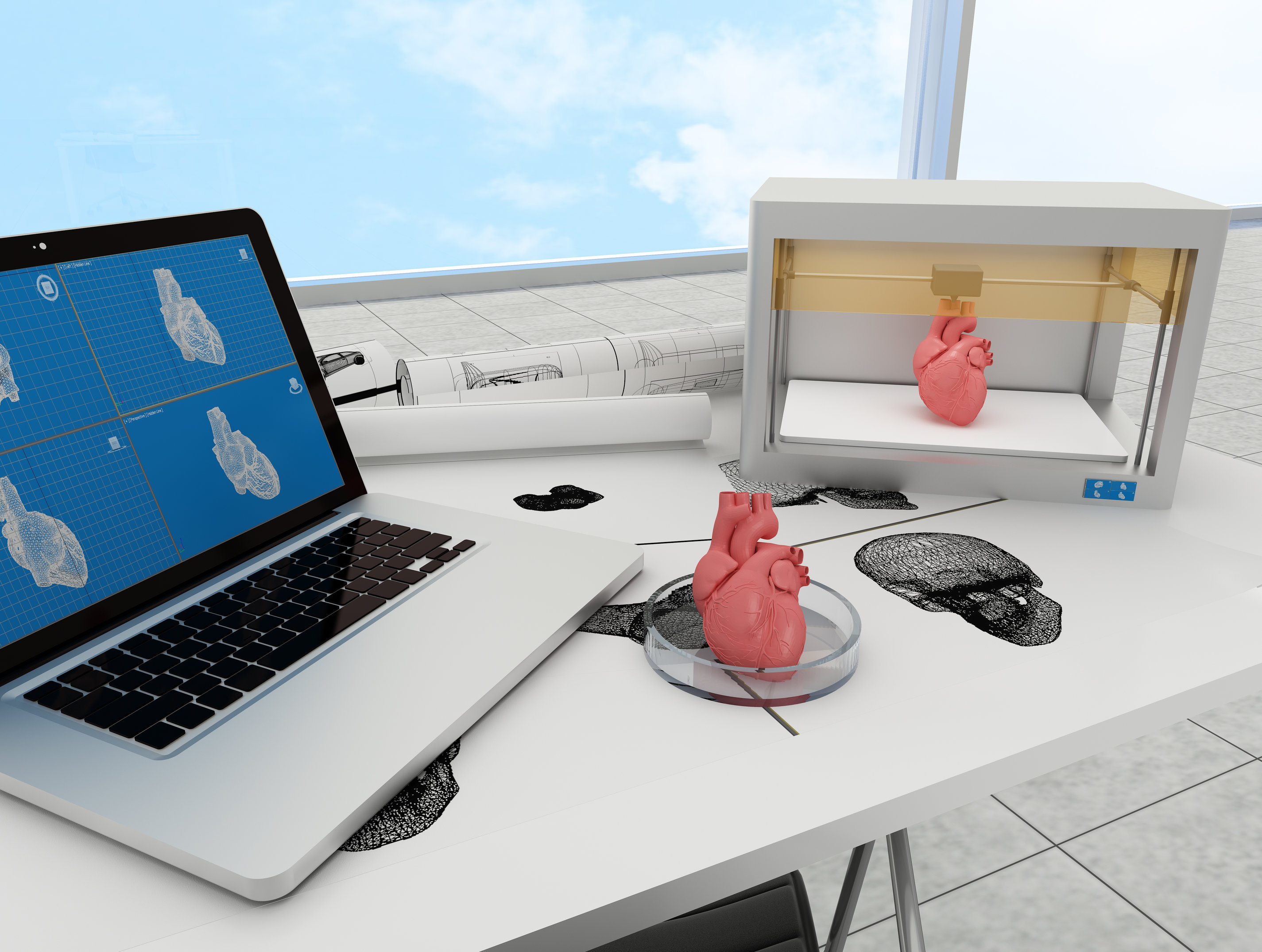
[[File:Muc16 dafuture.jpeg|thumb|right|320px| '''Figure 1:''' A vision of bioprinting in the future: Designing and printing whole organs in the lab.]] | [[File:Muc16 dafuture.jpeg|thumb|right|320px| '''Figure 1:''' A vision of bioprinting in the future: Designing and printing whole organs in the lab.]] | ||
Revision as of 01:33, 1 December 2016
biotINK - a synthetic biology approach to bioprinting
While interactions of biomolecules make up the cellular system, the three-dimensional organization of cells makes up tissues and organs. For decades now, scientists have tried to reconstitute such functional tissues by assembling the cells they are made out of - especially for the field of regenerative medicine, where the acquisition of suitable transplants may mean life or death for the patient. The prospect of creating personalized transplants artificially has since motivated groups all around the world to take part in this endeavour.[1]
But not only could bioprinting prove fruitful in the field of personalized medicine - for pharmacological applications, three-dimensional cell culture systems often constitute the first step in testing a potential pharmacological agent. Systems that resemble the human system as much as possible are hereby especially desirable, as they allow a more faithful prediction of a drug's effect in vivo and may furthermore reduce the need for lab animals.[2]
Bioprinting systems nowadays have not been able to have a distinct impact on either of these fields. Most of the current techniques here rely on a scaffold, on which cells are seeded. After a certain timespan of in vitro maturation, cells form intercellular contacts and start to grow into two-dimensional cell layers. For the reconstruction of the tissue, layers have to then be combined and the scaffold needs to be removed or degrade automatically within the recipient. The technical complexity of the printing process itself makes bioprinting as it exists now not only technically complex but also time-consuming and expensive. Furthermore, the growth of two-dimensional cellular layers does not resemble histogenesis in vivo and is limited to simple tissues that do not require exact positioning or multiple cell types.[3]
Our ambitious project intends to eliminate these distinct disadvantages: We are able to create three-dimensional cellular structures easily, quickly and at low cost by immediately cross-linking cells into a protein-cell-matrix upon printing. The interactions between cells with each other and the protein matrix are hereby mediated by the strongest non-covalent interaction found in nature - the biotin-streptavidin interaction. By using a two-component system of genetically engineered cells and proteins, we create a kind of molecular superglue that allows precise positioning of cells via bioprinting while locking them in position, allowing the formation of three-dimensional intercellular contacts and physiological microenvironments.
For delivery of cells, we hijacked a conventional 3D-printer that can be programmed to inject cells with a millimeter precision. Due to the quick nature of the polymerization of cells, we achieve a very high spatial resolution. But that's not everything: The printing system we created can easily be created by any lab around the world as long as they have a conventional 3D-printer, allowing to print cellular structures as required with only little initial investment.
Apart from the more medically orientated aspects of regenerative medicine and pharmacology, cellular systems are also of great interest for basic and applied research. While much on the molecular interactions within cells is known, comparatively little is known about the complex interactions of cells with each other. Thus, by providing a tool to make research on a supercellular scale more accessible, we are convinced that our system is able to noticeably advance science in many areas.
In addition to establishing the biotINK system itself, we have also worked on several ways to functionalize tissue: For the creation of synthetic organs, we modified cells to secrete insulin in response to external stimuli with infrared light. Also, we have worked on inducing vascularization in printed tissue in response to hypoxia. Furthermore, we have also modified our system to work in bacterial cells as well.
References
- ↑ Murphy, S. V., & Atala, A. (2014). 3D bioprinting of tissues and organs. Nature biotechnology, 32(8), 773-785.
- ↑ Griffith, L. G., & Swartz, M. A. (2006). Capturing complex 3D tissue physiology in vitro. Nature reviews Molecular cell biology, 7(3), 211-224.
- ↑ Derby, B. (2012). Printing and prototyping of tissues and scaffolds. Science, 338(6109), 921-926.