Composite parts
Nano-lantern(cAMP-1.6) regulated by ADH1 promoter (BBa_K1969007)
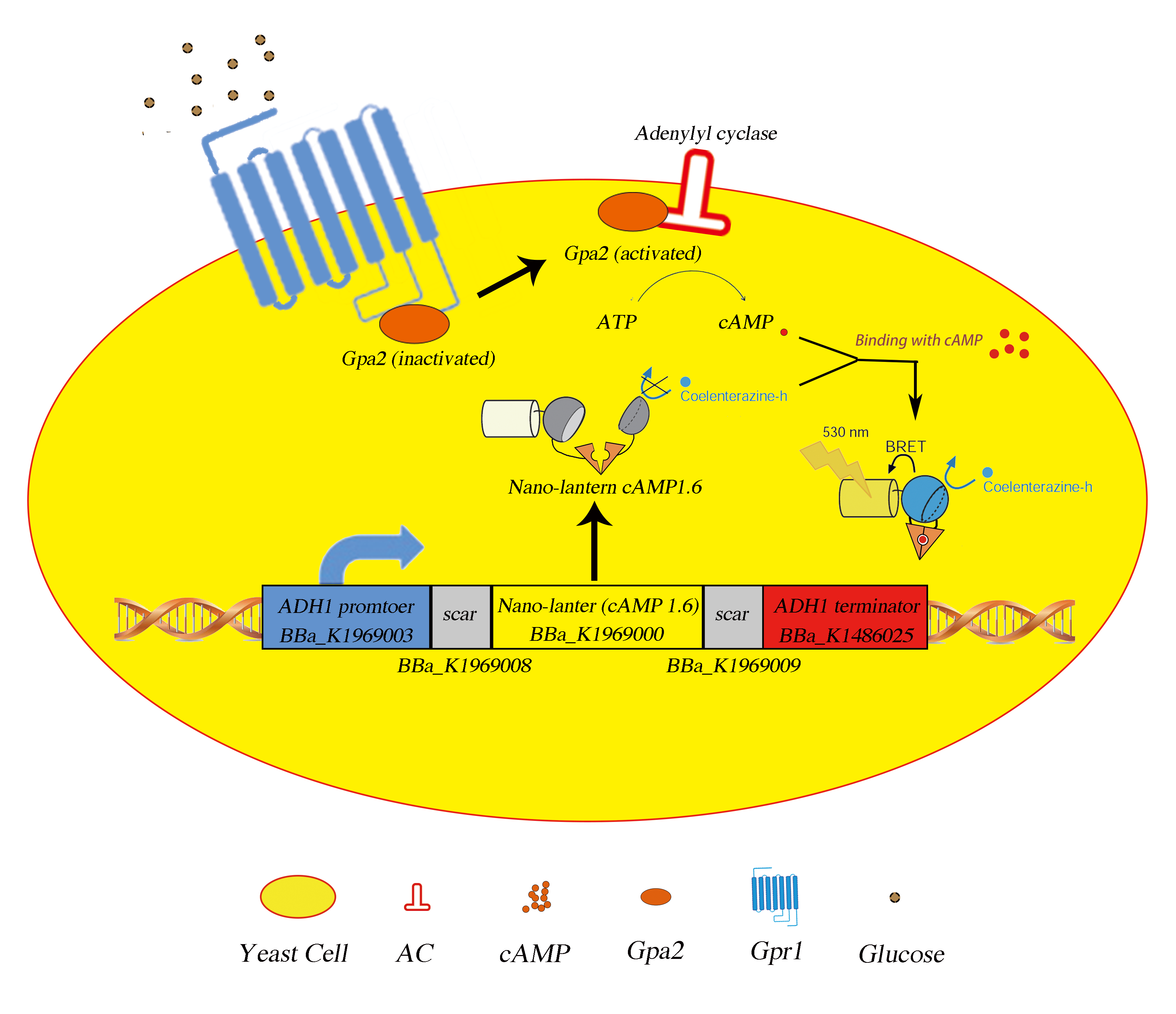
Fig.1 The picture of mechanism. Upon binding with extracellular glucose, the Gpa2 protein will disassociate from Gpr1 and activates adenylyl cyclase, yielding cAMp surge which can be detected by our composite part product.
Nano-lantern(cAMP-1.6) contains a portion of enhance YFP with 10 amino acids deleted at C terminus, denoted as Venus△C10, a portion of mutated Renilla luciferase with 3 amino acids deleted at N terminus, Rluc8, and a cAMP binding domain of EPAC1 (Exchange Protein Directly Activated by cAMP) flanked by two separate parts of Rluc8. Upon binding with the cAMP molecule, the catalytic activity of the split luciferase will increase as the separate parts are brought together because of the conformational change in EPAC, thus results in the change in luminescence via the BRET effect. Thus this part can tell us the relative concentration of extracellular ligands.
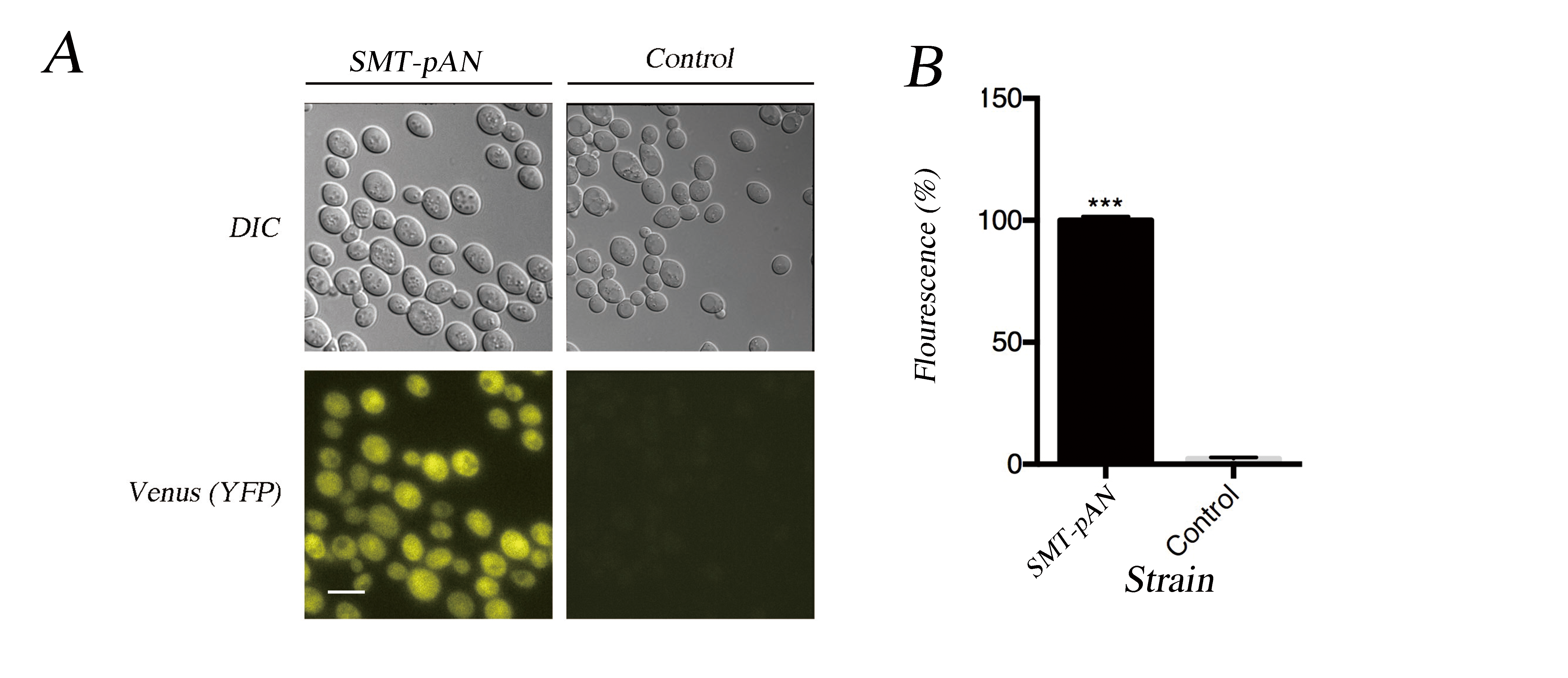
Fig.2 Characterization of expressed Nano-lantern(cAMP 1.6). (A) The fluorescent microscopy results together with the DIC result show the successful expression of NLc in transformants. Scale bar: 10μm; (B) The florescence of transformants was quantified using ImageJ, the value was the average difference between the yeast cell and background under three different fields and normalized with the fluorescence of pADH1-NLc. Data was analyzed using Student-t test, *** indicates p < 0.001.
As the cAMP surge in yeast triggered by GPCR sensing mechanism is incredibly fast and decays within 5 minutes, we monitor the glucose response in first 5 minutes. The results shows that the luminescence in engineered strain was obviously higher than control group (Fig.3). This part showed a good positive correlation between luminescence and the concentration of glucose, and we may need more experiment groups to improve the statistical power as the p values of one-way ANOVA was quite confusing in most of the experimental groups. As the results show, strain SMT-pAN has a good relationship between luminescence and glucose concentration at 0-1min (Fig.3).
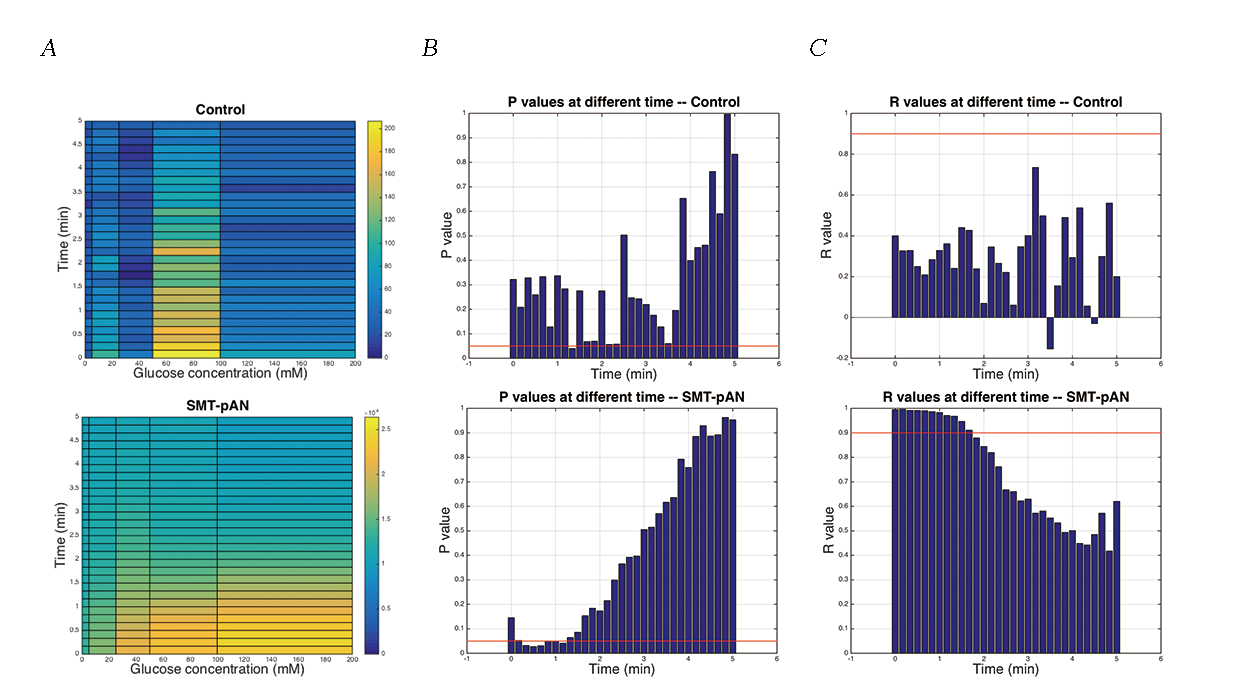
Fig.3 The luminescence of transformants after glucose stimulation. (A) Dose dependent effects of glucose exposure on cAMP mediated luminescence measured simultaneously in 96-well plate format. Data was shown as average of three parallel groups. The pseudo-color scale indicates intensities of emitted luminescence signals; (B) P values calculated by One-way ANOVA at different time points within three parallel groups, the red line indicated the value of 0.05; (C) The R values calculated by linear regression of the average luminescence at different time points and the the logarithm of the glucose concentration (except 0mM) to base 10. Red line indicated the value of 0.9.
In order to detect the changes in glucose concentration in pathologic urine samples, we added corresponding amount of glucose into urine samples collected from a healthy individual to make mimic pathologic urine samples with glucose concentration of 0, 10, 50 mM. As the P value was the least at 30s in strain SMT-pAN, we compared the glucose response at 30s after adding mimic pathologic urine samples (Fig.4). The results showed postitive correlation between three strains. Although the significance between 10mM treatment groups and the control groups is not promising, we found our device capable of differentiate mimic pathologic urine samples containing 50mM glucose and the healthy urine.
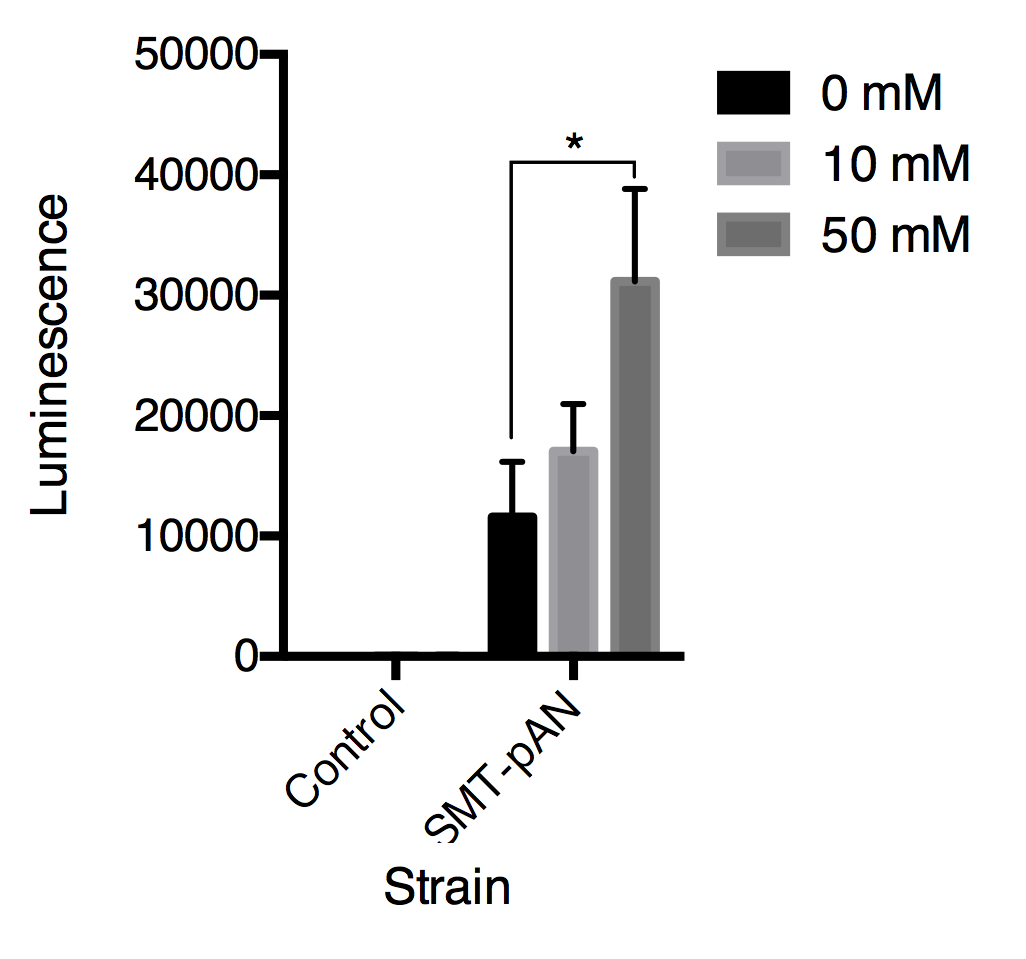
Fig.4 Luminescence triggered by mimic pathologic urine samples with gradient glucose concentration. All data are presented as mean ± SD, n=3. Significance was analyzed using Student-t test.
ADRB2 with His-tag regulated by ADH1 promoter (BBa_K1969020)
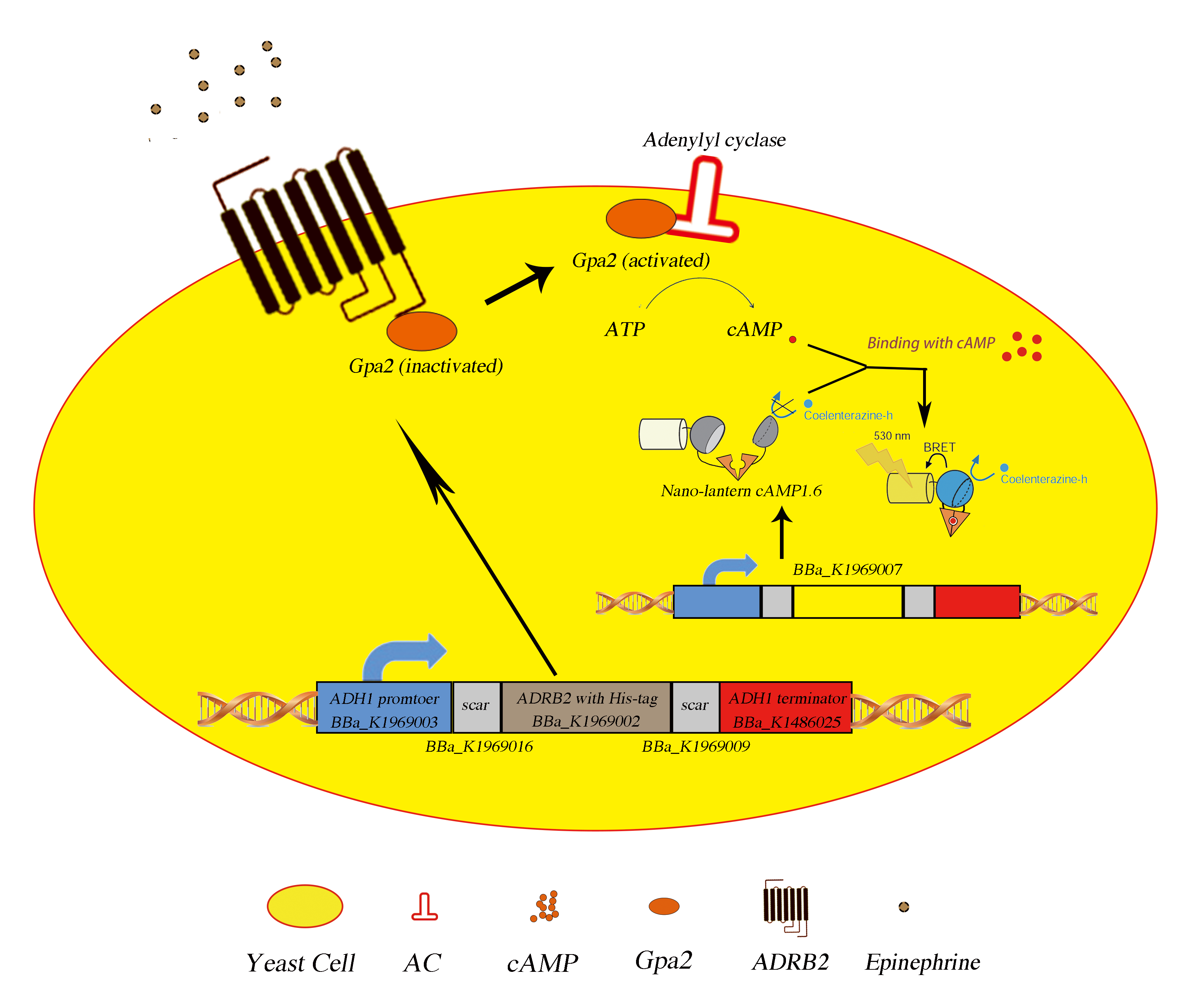
Fig.5 The picture of mechanism. Upon binding with extracellular epinephrine, the Gpa2 protein will disassociate from ADRB2 (putatitive) and activates adenylyl cyclase, yielding cAMP surge which can be detected by our composite part (BBa_K1969007) product.
We expressed the human adrenergic beta 2 downstream the ADH1 promoter and transformed into strain SMT-pAN to generate a epinephrine sensing strain, SMT-pANpAA. Actually, the cds for ADRB2 has already been registered as a biobrick part (BBa_K209472), while it’s not biobrick compatitble, so we use site mutation to make it a biobrick compatitble one (BBa_K1969001) and added a His-tag to its C terminus (BBa_K1969002).
We did immunocytochemistry experiments to test the localization of the membrane protein ADRB2. The results showed that the signal of the second antibody was mainly located on the cell membrane, which indicated that heterologously expressed ADRB2 was successfully located on cell membrane.
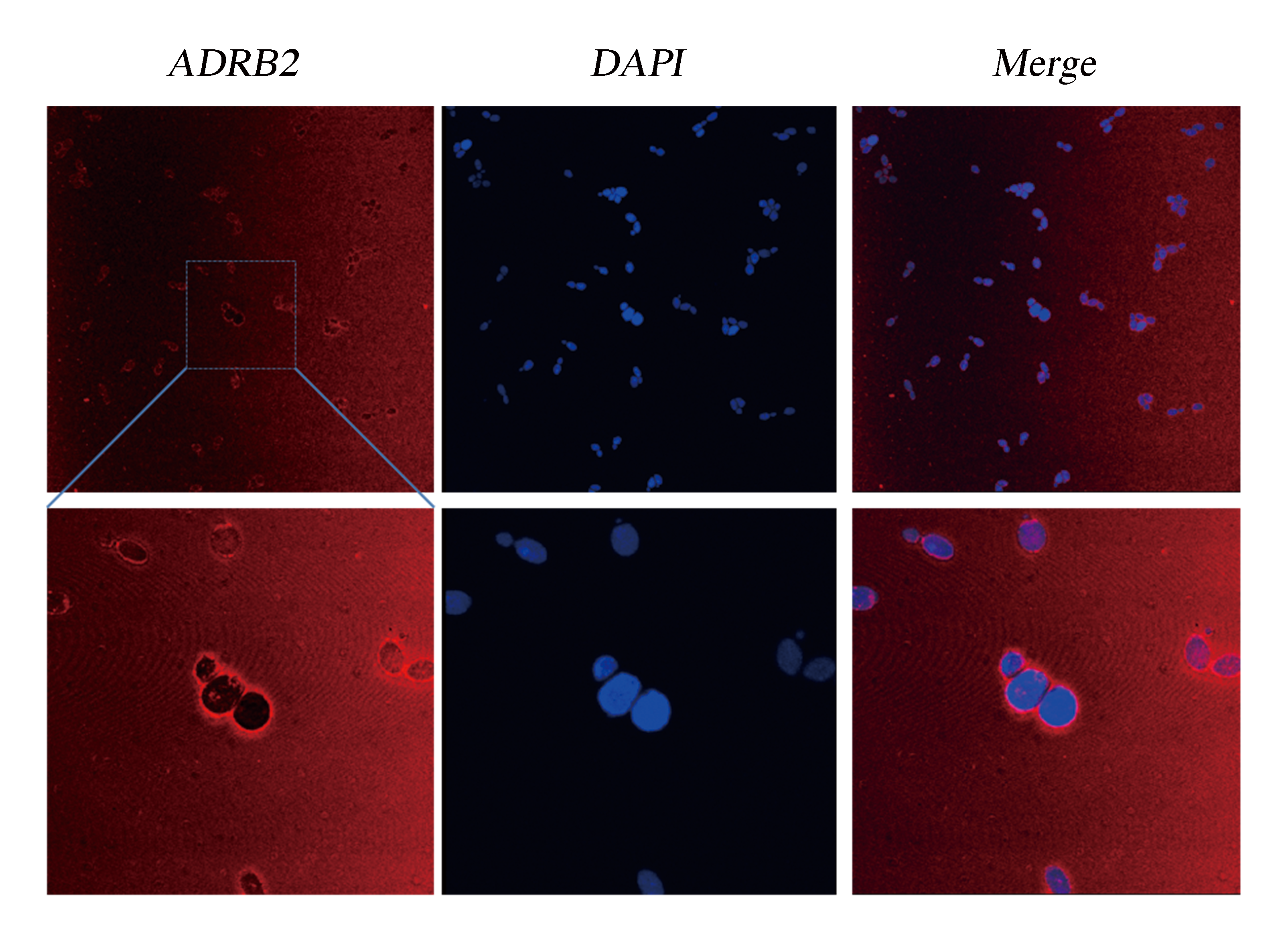
Fig.6 Detection of Membrane Localization of ADRB2 by Immunocytochemistry. The yeast cells were stabilized and stained with DAPI. As ADRB2 is expressed with a His-tag at C' terminal, thus anti-His mAb is used as the primary antibody and dunkey anti-mouse IgG 594 (Invitrogen) as the second antibody. Pictures were taken with Leica-SP5, 63 X.
Although the functional coupling of ADRB2 with yeast endogenous pheromone sensing pathway has been well studied in past few decades, the feasibility of functional signal transduction via ADRB2-Gpa2 has never been tested[4]. So we used our engineered strains SMT-pANpAA as biosensors to detect extracellular epinephrine concentration in PBS solution. The response of the epinephrine is detected within first 5 minutes after addition of epinephrine (dissolved in HAc then diluted using PBS) (Fig.7).
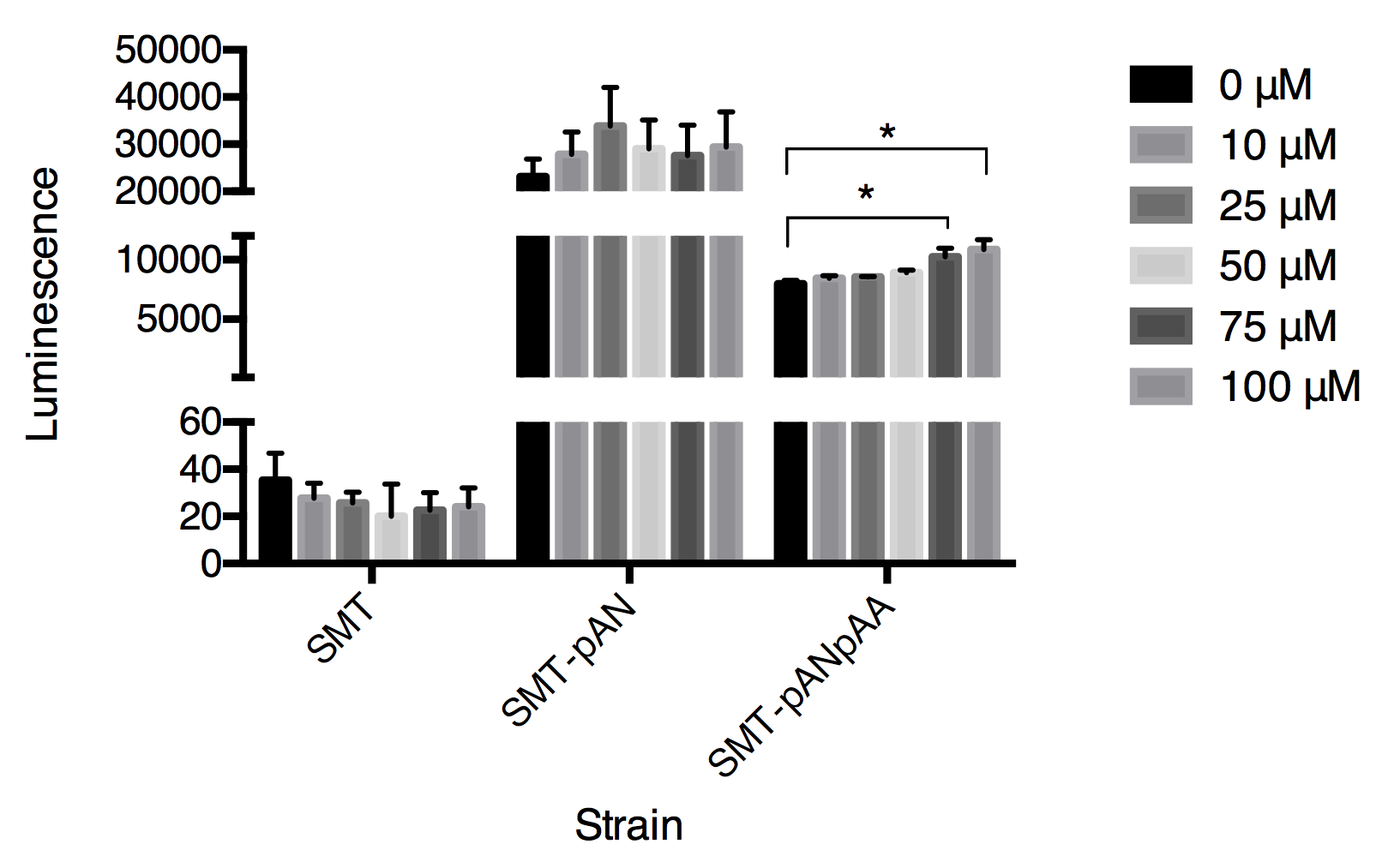
Fig.7 The luminescence triggered by epinephrine detected in three strains. Luminescence was collected at 30s after adding epinephrine. Data expressed as mean ± SD, significance was analyzed using Student-t test, n=3.
In this graph, we can tell that the constructed strain SMT-pANpAA was able to differentiate solution with relatively high concentration of epinephrine (75-100μM) with P value less than 0.05 by Student-t test. Although the difference between 100 μM and 75 μM treatment group was significant, the device can’t differentiate epinephrine solution at low concentration. And the expression of two exogenous protein under the same constitutive promoter ADH1 seems to interfere with each other as the overall luminescence decrease in strain SMT-pANpAA compared with strain SMT-pAN.




